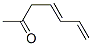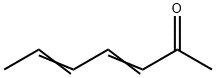
2,4-HEPTADIEN-6-ONE synthesis
- Product Name:2,4-HEPTADIEN-6-ONE
- CAS Number:3916-64-1
- Molecular formula:C7H10O
- Molecular Weight:110.15
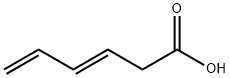
67-64-1
0 suppliers
$21.60/10ml

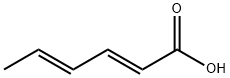
123-73-9
228 suppliers
$40.00/5g

3916-64-1
40 suppliers
$65.00/100mg
Yield:3916-64-1 91.3%
Reaction Conditions:
with sodium hydroxide in water at 30 - 40; for 6 h;Aldol Condensation;
Steps:
1 Example 1: 3,5-heptadien-2-one (compound of formula (I)):
The reaction flask was charged with 639 g (11 mol) of acetone and 500 ml of 8% aqueous sodium hydroxide. The mixture was stirred at room temperature (20-25 ° C.) for 30 minutes and slowly added to 70.1 g (1 mol) of crotonaldehyde The dropping temperature was controlled between 30-40 . After the completion of the dropwise addition, the reaction was continued at 30-40 for 6 hours. The reaction was completely followed by GC (crotonaldehyde ≤2%) and the reaction solution was cooled to room temperature (20-25 ), 500ml of water and 1000ml of toluene was added and stirred for 30 minutes, allowed to stand for 30 minutes, separated into layers, and then 500ml of water was added to the organic layer and stirred for 30 minutes, allowed to stand for 30 minutes, The organic layer was depressurized and desolvented. Toluene was recovered and applied to give 102.8 g of a light yellow liquid. The purity of the gas phase was 97.8%. The yield of pure organic compound was 91.3%.
References:
CN107200701,2017,A Location in patent:Paragraph 0059; 0060; 0061; 0062; 0063; 0064; 0065