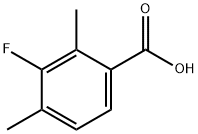
2,4-DIMETHYL-3-FLUOROBENZOIC ACID synthesis
- Product Name:2,4-DIMETHYL-3-FLUOROBENZOIC ACID
- CAS Number:26583-81-3
- Molecular formula:C9H9FO2
- Molecular Weight:168.16
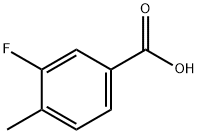
350-28-7
280 suppliers
$8.00/1g

74-88-4
349 suppliers
$15.00/10g

26583-81-3
22 suppliers
$320.00/250mg
Yield:26583-81-3 2.18 g
Reaction Conditions:
Stage #1: 3-fluoro-4-methyl-benzoic acidwith 2,2,6,6-tetramethyl-piperidine;n-butyllithium in tetrahydrofuran;hexane at -78 - 0; for 2.25 h;
Stage #2: iodomethane in tetrahydrofuran;hexane at 20;
Steps:
3.A Step A: Preparation of 3-fluoro-2,4-dimethyl-benzoic acid
To a stirred solution of n-butyllithium (1.6 M solution in hexanes, 18 mL, 29 mmol) in anhydrous tetrahydrofuran (40 mL) at 0 °C was slowly added 2,2,6,6-tetramethylpiperidine (4.9 mL, 29 mmol). The mixture was stirred for 15 minutes then was cooled to -78 °C and a solution of 3-fluoro-4-methyl-benzoic acid (2.0 g, 13 mmol) in anhydrous tetrahydrofuran (10 mL) was added dropwise. The reaction mixture was stirred at -78 °C for 1.5 h, then warmed to -50 °C and stirred for an additional 45 min. Iodomethane (3.2 mL, 52 mmol) was then added slowly and the reaction mixture was allowed to warm to room temperature and stirred overnight. Water was added and the mixture was washed with diethyl ether. The aqueous phase was acidified with 6 N hydrochloric acid to pH 2 and extracted with diethyl ether (x2) then the combined organic extracts were washed with brine (x1), dried over sodium sulfate and concentrated under reduced pressure to afford the title compound as a pale yellow solid (2.18 g), which was used without further purification in the next step. 1H NMR (CDCl3) δ 7.75 (d, 1H), 7.10-7.07 (m, 1H), 2.55 (d, 3H), 2.33 (d, 3H).
References:
WO2022/26500,2022,A1 Location in patent:Page/Page column 62
53874-26-3
56 suppliers
$65.00/50mg

26583-81-3
22 suppliers
$320.00/250mg

124-38-9
129 suppliers
$214.00/14L
26584-26-9
25 suppliers
inquiry

26583-81-3
22 suppliers
$320.00/250mg