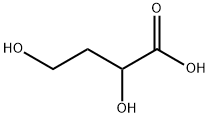
2,4-dihydroxy-Butanoic acid synthesis
- Product Name:2,4-dihydroxy-Butanoic acid
- CAS Number:1518-62-3
- Molecular formula:C4H8O4
- Molecular Weight:120.1
1492-23-5
40 suppliers
$81.79/25mg

1518-62-3
13 suppliers
$159.00/10mg
110-15-6
951 suppliers
$5.00/10g
Yield:-
Reaction Conditions:
with nitrogen(II) oxide in waterInert atmosphere;
Steps:
13-1 Preparation of hydroxy-gamma butyrolactone and dialkyl succinate from O-succinyl homoserine
The direct deamination and hydrolysis reaction of O-succinyl homoserine (O-SH) was carried out as shown in FIG. 14, and specifically performed as follows.First, water was used as a solvent. However, since the solubility of O-SH in water is 8.7 g / L, if O-SH is added in the initial stage of the reaction, the inert gas (N2, Ar, etc.) Since bubbles are generated by the generated nitrogen gas, a small amount of the bubbles are introduced several times. The amount of O-SH finally added was 250 g, and the concentration was 50 wt% based on O-SH.The results of analyzing the product of the aqueous solution after completion of the reaction are shown in Table 34 below.As shown in Table 34, it was confirmed that succinic acid was produced by hydrolysis of O-SH and 2,4-dihydroxy-butanoic acid produced by the desamination reaction of DL-HS was produced. Also, it was confirmed that the precipitate formed in the final product was succinic acid and the solubility in water was 58 g / L (20 DEG C), so that a part of the succinic acid produced by the reaction was present in the aqueous solution in the undissolved state.About 64% of succinic acid with 88.5% purity was recovered through a first-order filter process, and succinic acid with a purity of 99.5% was recovered by further washing with water. An aqueous solution of 2,4-dihydroxy-butanoic acid containing 16.6% succinic acid was obtained in the filtrate from which the succinic acid was removed in the first filter process.
References:
CJ CHEILJEDANG CORPORATION;KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY;YANG, YOUNG RYEOL;KIM, BYUNG SIK;KIM, JEONG HYUN;LEE, JUNG HO;SHIN, HYUN KWAN;KIM, JU NAM;CHO, KYUNG HO KR2015/118287, 2015, A Location in patent:Paragraph 0286-0292
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1518-62-3
13 suppliers
$159.00/10mg
766-92-7
125 suppliers
$51.00/5g
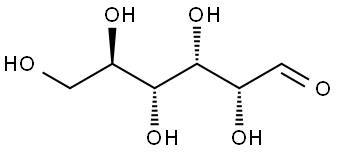
50-99-7
995 suppliers
$6.00/25g

1518-62-3
13 suppliers
$159.00/10mg
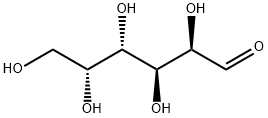
59-23-4
641 suppliers
$6.00/25g

1518-62-3
13 suppliers
$159.00/10mg
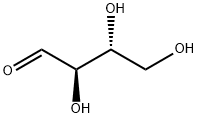
583-50-6
137 suppliers
$42.00/200mg

1518-62-3
13 suppliers
$159.00/10mg