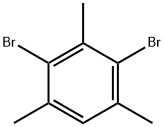
2,4,6-Trimethybromombenzene synthesis
- Product Name:2,4,6-Trimethybromombenzene
- CAS Number:576-83-0
- Molecular formula:C9H11Br
- Molecular Weight:199.09
Yield:576-83-0 73%
Reaction Conditions:
with bromine in tetrachloromethane;Product distribution / selectivity;
Steps:
1
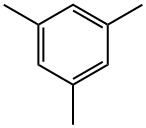
Example 1; Bromination of Mesitylene Using Elemental Bromine:; The bromination of mesitylene (1) using elemental bromine was carried out in a static micromixer (Technical University of Ilmenau, Faculty of Machine Construction, Dr. -Ing. Norbert Schwesinger, P.O. Box 100565, D-98684, Ilmenau) having a physical size of 0.8 mm×0.8 mm×0.6 mm and a total volume of 0.125 μl and a total pressure loss of about 1000 Pa. The static micromixer was connected to a Teflon capillary having an internal diameter of 0.25 mm and a length of 1 m via an outlet and an Omnifit medium-pressure HPLC connector (Omnifit, Germany). The temperatures of the static micro-mixer and the Teflon capillary were regulated in an water-filled jacketed vessel thermostatted to 10° C.In order to prepare a solution of mesitylene, 1.2 g (0.01 mol) of mesitylene were diluted with tetrachloromethane to a total volume of 2 ml. In order to prepare a solution of elemental bromine, 1.7 g (0.011 mol) of bromine were diluted with tetrachloromethane to a total volume of 2 ml. The two solutions were subsequently transferred into the static micromixer using a metering pump (Harvard Apparatus Inc., Pump 22, South Natick, Mass., USA) and 2 ml polypropylene syringes (B. Braun Melsungen AG, Germany). The flow rate here was set to 10 ml/min. The mixed reaction solution was subsequently passed into 2 ml of an HPLC buffer solution comprising acetonitrile and 1% trifluoroacetic acid in the ratio 1:1 (Merck, Darmstadt) in order to terminate the bromination reaction. The reaction mixture was evaluated by combined GC/MS analysis. The reaction mixture comprised 88 area-% of the chromatogram of the monobrominated product (2), 9 area-% of the dibrominated product (3) and 3 area-% of the unbrominated mesitylene (1).In order to determine the preparative yield of the brominated reaction products, the mixed reaction solution was stirred into a beaker containing 50 ml of water. The system comprising static micromixer and Teflon capillary was subsequently rinsed firstly with 10 ml of water and subsequently with 10 ml of dichloromethane. The combined liquid phases were then stirred for 20 minutes and subsequently extracted three times with 20 ml of diethyl ether each time. The combined ethereal extracts were dried over magnesium sulfate and freed from solvent under reduced pressure, giving 1.7 g (corresponding to 73% of the theoretical yield) of a brownish oil, whose content of monobrominated product (2) was determined by combined GC/MS analysis as 85 area-% of the chromatogram.; Example 2; The set-up and performance were as in Example 1, but the flow rate was set to 20 μl/min. Combined GC/MS analysis of the reaction mixture obtained in this way gave a composition of 51 area-% of the chromatogram of the mono-brominated product (2), 47 area-% of the dibrominated product (3) and 2 area-% of mesitylene brominated in the methyl side chain.
References:
US7271302,2007,B1 Location in patent:Page/Page column 5-6
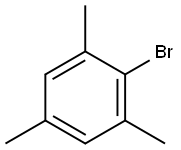
108-67-8
379 suppliers
$5.00/5G

576-83-0
256 suppliers
$27.00/25G

108-67-8
379 suppliers
$5.00/5G
27129-86-8
149 suppliers
$14.00/1g

576-83-0
256 suppliers
$27.00/25G
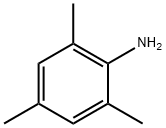
88-05-1
340 suppliers
$6.00/1g

576-83-0
256 suppliers
$27.00/25G
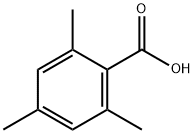
480-63-7
329 suppliers
$6.00/5g

576-83-0
256 suppliers
$27.00/25G