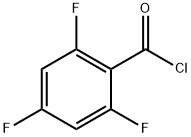
2,4,6-TRIFLUOROBENZOYL CHLORIDE synthesis
- Product Name:2,4,6-TRIFLUOROBENZOYL CHLORIDE
- CAS Number:79538-29-7
- Molecular formula:C7H2ClF3O
- Molecular Weight:194.54
28314-80-9
316 suppliers
$6.00/1g

79538-29-7
167 suppliers
$12.00/1g
Yield:79538-29-7 100%
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 0 - 25; for 3 h;Inert atmosphere;
Steps:
1,3-Difluoro-5,6-dihydroxy-9H-xanthen-9-one (29)
To a dried 100 mL round-bottomed flaskwas added 2,4,6-trifluorobenzoic acid (26) (1.00 g, 5.68 mmol). The flask containing 2,4,6-trifluorobenzoic acid (26) and a magnetic stir bar was placed under high vacuum for about 10min. The flask was carefully sealed and CH2Cl2 (25 mL) was added by using a syringe underargon. The flask was then placed in an ice bath. To the stirring solution of 2,4,6-trifluorobenzoicacid (26) under argon at 0 °C was added oxalyl chloride (0.98 mL, 11.4 mmol) dropwise viasyringe, followed by a catalytic amount of DMF. The ice bath was removed and the reactionmixture was stirred at room temperature for 3 hours. The reaction mixture was concentrated by rotary evaporation under argon to yield 2,4,6-trifluorobenzoyl chloride (27) (1.10 g, 100%) as acolorless oil.
References:
Chantarasriwong, Oraphin;Milcarek, Andrew T.;Morales, Theodore Habarth;Settle, Aspen L.;Rezende, Celso O.;Althufairi, Bashayer D.;Theodoraki, Maria A.;Alpaugh, Mary L.;Theodorakis, Emmanuel A. [European Journal of Medicinal Chemistry,2019,vol. 168,p. 405 - 413] Location in patent:supporting information