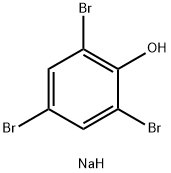
2,4,6-TRIBROMOPHENOLSODIUMSALT synthesis
- Product Name:2,4,6-TRIBROMOPHENOLSODIUMSALT
- CAS Number:2666-53-7
- Molecular formula:C6H4Br3NaO
- Molecular Weight:354.8
108-95-2
746 suppliers
$14.00/25g

2666-53-7
2 suppliers
inquiry
Yield:-
Reaction Conditions:
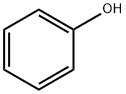
Stage #1: phenolwith bromine chloride;iron in ethyl bromide at 17 - 28; for 5 h;
Stage #2: with sodium ethanolate in ethanol;Solvent;Temperature;Reagent/catalyst;
Steps:
1.1; 2.1; 3.1; 4.1; 5.1 1) Synthesis of an ethanol solution of sodium 2,4,6-tribromophenolate:
Add 94g of phenol to the four-necked bottle,Organic solvent bromoethane 658g,Adding catalyst I iron powder2.4g,When the reaction temperature is controlled at 17 ° C (control reaction temperature does not exceed 20 ° C),After the addition of bromine chloride brominating agent 351.2g,After heating to 28 ° C, the reaction was kept for 5 h.The catalyst I is removed by filtration, and the organic solvent is distilled and recovered, and the remaining product is tribromophenol;Then, the tribromophenol was added to 527.5 g of an ethanol solution having a mass concentration of 15% sodium ethoxide, and the reaction was completed to obtain an ethanol solution of sodium 2,4,6-tribromophenolate.
References:
CN109053389,2018,A Location in patent:Page/Page column 4-6

118-79-6
370 suppliers
$5.00/25g

2666-53-7
2 suppliers
inquiry
