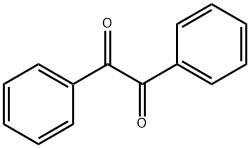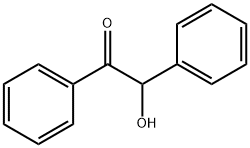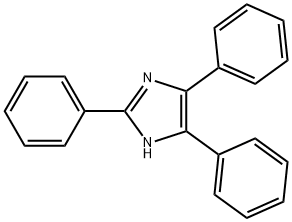
2,4,5-Triphenylimidazole synthesis
- Product Name:2,4,5-Triphenylimidazole
- CAS Number:484-47-9
- Molecular formula:C21H16N2
- Molecular Weight:296.37
Synthesis of 2,4,5-Triphenylimidazoles Novel Mannich Bases as Potential Antiinflammatory and Analgesic Agents
DOI: 10.3923/crc.2012.99.109
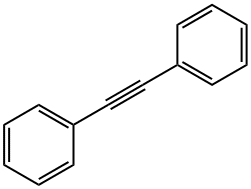
501-65-5
215 suppliers
$16.00/5g
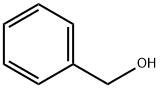
100-51-6
1401 suppliers
$5.00/100g

484-47-9
197 suppliers
$13.00/1g
Yield:484-47-9 86%
Reaction Conditions:
Stage #1:diphenyl acetylene;benzyl alcohol with iodine;dimethyl sulfoxide at 130; for 24 h;Green chemistry;
Stage #2: with ammonium acetate in ethanol at 100; for 2 h;Green chemistry;Solvent;
Steps:
4.1 General procedure for the synthesis 2,4,5-trisubstituted imidazoles (3)
General procedure: Alkyne (0.5 mmol, 1 equiv.), primary alcohol (0.5 mmol, 1 equiv.), I2 (1 mmol, 253.8 mg, 2 equiv.) and DMSO (2 mL) were mixed in a round bottom flask and heated at 130 °C for 24 h. Thereafter, ammonium acetate (5 mmol, 385.4 mg, 10 equiv.) and EtOH (2 mL) was added to the mixture and heated at 100 °C for 2 h. After cooling, a solution of 1% Na2S2O3 was added dropwise to the mixture to form a precipitate which was filtered and dried. The crude product was recrystallized from EtOH to afford spectroscopically pure imidazole 3.
References:
Jeena, Vineet;Naidoo, Shivani [Tetrahedron,2020] Location in patent:supporting information
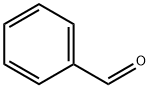
100-52-7
954 suppliers
$17.30/2g

484-47-9
197 suppliers
$13.00/1g

501-65-5
215 suppliers
$16.00/5g
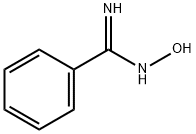
613-92-3
200 suppliers
$5.00/250mg

484-47-9
197 suppliers
$13.00/1g