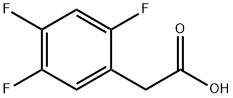
2,4,5-Trifluorophenylacetic acid synthesis
- Product Name:2,4,5-Trifluorophenylacetic acid
- CAS Number:209995-38-0
- Molecular formula:C8H5F3O2
- Molecular Weight:190.12
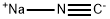
773837-37-9
0 suppliers
inquiry

75-09-2
1239 suppliers
$10.00/25ML
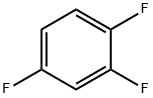
367-23-7
382 suppliers
$8.00/5g

209995-38-0
521 suppliers
$10.00/10g
Yield:209995-38-0 99.2%
Reaction Conditions:
Stage #1: sodium cyanidewith sodium stearate;nitrobenzene;sodium hydroxide in water at 115; under 3420.23 Torr; for 0.75 h;Inert atmosphere;
Stage #2: dichloromethane;1,2,4-trifluorobenzene at 6 - 142; under 2280.15 - 5320.36 Torr; for 6 h;Further stages;Solvent;Pressure;Reagent/catalyst;
Steps:
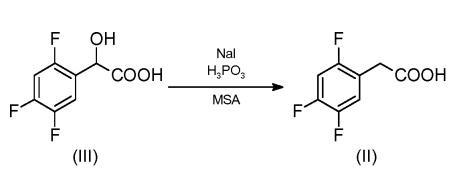
1 Example 1A method for synthesizing 2,4,5-trifluorophenylacetic acid, comprising the following steps:
1) Sodium cyanide,Sodium hydroxide,Mix sodium stearate and deionized water,Obtaining the mixture M1, sodium cyanide,Sodium hydroxide,The molar ratio of sodium stearate to deionized water was 1:0.16:0.07:13.2) Under argon protection,Mix the catalyst with nitrobenzene,Control the reaction temperature to 115 ° C,The reaction pressure is 4.5 atmospheres.After stirring for 45 minutes,First add dichloromethane,When the amount of dichloromethane added is 5% of its own,Start adding 1,2,4-trifluorobenzene,The volume ratio of 1,2,4-trifluorobenzene and dichloromethane drops was kept equal.After the drop is over,Control the reaction temperature to 142 ° C,The reaction pressure is 7 atmospheres.The reaction was carried out for 1.5 h to obtain a mixture M2.Control the reaction temperature to 6 ° C,The reaction pressure is 3 to 4 atmospheres.Add the mixture M1, drop,Control the reaction temperature to 76 ° C,The reaction pressure is 6 atmospheres.Continue to react at 4.5h,After cooling and dropping to normal temperature, a mixture M3 was obtained.In this step,The molar ratio of 1,2,4-trifluorobenzene to dichloromethane is 1:1.02,The ratio of 1,2,4-trifluorobenzene to nitrobenzene is 1 g: 4.5 mL.The molar ratio of 1,2,4-trifluorobenzene to sodium cyanide is 1:1.06,The mass ratio of 1,2,4-trifluorobenzene to the catalyst was 1:0.18.The preparation method of the catalyst used in this step is as follows:HY-98 zeolite,Zinc chloride is added to a 5% by mass aqueous solution of hydrochloric acid,Soak for 3h, then concentrate and evaporate with a rotary evaporator.The obtained solid is baked and activated at 200 ° C for 7 h to obtain zinc chloride.The mass ratio of zeolite to aqueous hydrochloric acid was 1:4:13.3) The mixture M3 is filtered to remove insoluble matter, layered, and the organic phase is washed with water.After drying anhydrous sodium sulfate,The solvent was evaporated to give a liquid L.4) Liquid L,Tetrabutylammonium chloride is mixed with hydrochloric acid with a mass fraction of 20%.Control the reaction temperature to 108 ° C,The reaction pressure is 4 atmospheres.The reaction ended in 80 minutes.After cooling the system, pour it into crushed ice.A white solid 2,4,5-trifluorophenylacetic acid was precipitated.In this step,The mass ratio of liquid L to tetrabutylammonium chloride is 1:0.1;The mass ratio of liquid L to hydrochloric acid was 1:6.5.The molar yield was 99.2% and the GC purity was 98.7%.
References:
CN110128258,2019,A Location in patent:Paragraph 0033-0086
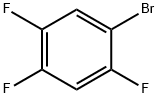
327-52-6
335 suppliers
$6.00/5g
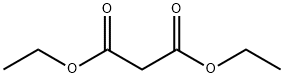
105-53-3
782 suppliers
$5.00/25g

209995-38-0
521 suppliers
$10.00/10g
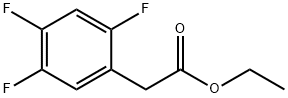
1256470-41-3
30 suppliers
$21.00/100mg

209995-38-0
521 suppliers
$10.00/10g
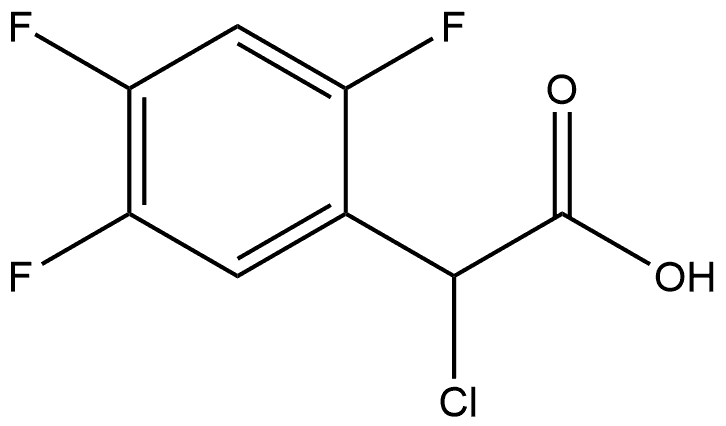
1036273-14-9
0 suppliers
inquiry

209995-38-0
521 suppliers
$10.00/10g
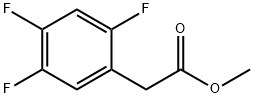
1036273-20-7
25 suppliers
inquiry

209995-38-0
521 suppliers
$10.00/10g