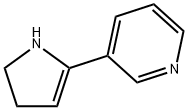
2-(3-Pyridinyl)-2-pyrroline synthesis
- Product Name:2-(3-Pyridinyl)-2-pyrroline
- CAS Number:1125-96-8
- Molecular formula:C9H10N2
- Molecular Weight:146.19
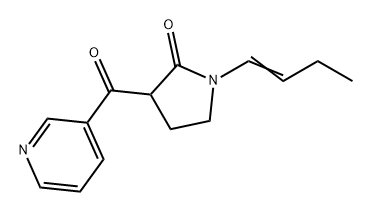
1308392-26-8
1 suppliers
inquiry

1125-96-8
4 suppliers
inquiry
Yield:1125-96-8 82%
Reaction Conditions:
with nitric acid at 5; for 8 h;Reagent/catalyst;
Steps:
3 Example 3
Add 150g of sodium ethoxide and 1000mL of methyl tert-butyl ether into a three-necked flask, install the magnet and condenser, and replace the system with nitrogen; replace 100g of methyl nicotinate and 117g of N-butenylpyrrolidone with 200mL of methyl tert-butyl ether the base is evenly mixed, the system is slowly heated to 50°C, and the mixed solution is added dropwise. After dropping, the reaction is kept for 3 hours, and then cooled to 5°C. 6N (ie: 6mol/L) nitric acid is added dropwise to quench the reaction and adjust the pH value. React for 8 hours until 4; add at the end of the reaction6N lye adjustmentWhen the pH value reached 10, dichloromethane was added for extraction three times; the organic phases were combined, dried, concentrated, and distilled to obtain 9.233 g of the light yellow oily enamine intermediate with a yield of 82%.
References:
CN112745294,2021,A Location in patent:Paragraph 0044-0045
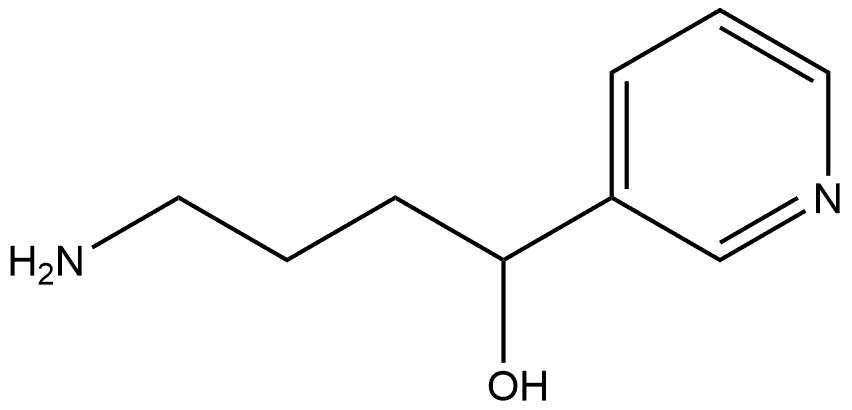
1226127-81-6
0 suppliers
inquiry

1125-96-8
4 suppliers
inquiry
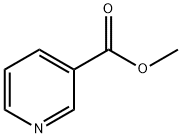
93-60-7
467 suppliers
$8.00/25g

1125-96-8
4 suppliers
inquiry