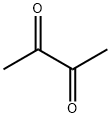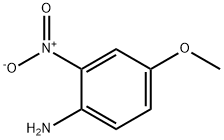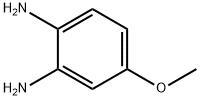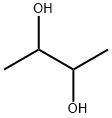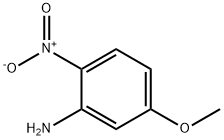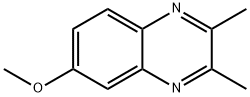
2,3-DIMETHYL-6-METHOXYQUINOXALINE synthesis
- Product Name:2,3-DIMETHYL-6-METHOXYQUINOXALINE
- CAS Number:6637-22-5
- Molecular formula:C11H12N2O
- Molecular Weight:188.23
Yield:6637-22-5 95%
Reaction Conditions:
with indium;indium(III) chloride in methanol; for 1.5 h;Reflux;Inert atmosphere;
Steps:
4.2. General procedure for the indium-mediated reductivecyclization of 2-nitroanilines to prepare quinoxaline
General procedure: 4.2. General procedure for the indium-mediated reductivecyclization of 2-nitroanilines to prepare quinoxalines A mixture of the 2-nitroaniline derivative (1.0 mmol), dione (1.0or 2.0 mmol), indium (0.574 g, 5.0 mmol), and acetic acid (0.300 g,5 mmol or 0.600 g,10 mmol) or indium chloride (0.221 g,1 mmol or0.265 g,1.2 mmol) in methanol (5 mL) or toluene (5 mL) was stirredat 50 C, 80 C, or reux under a nitrogen atmosphere. After com-pletion of the reaction, the reaction mixture was diluted with ethylacetate (30 mL), ltered through Celite, and the ltrate was pouredinto 10% NaHCO3 (30 mL) and extracted with ethyl acetate(30 mL3). The combined organic extracts were dried over MgSO4,ltered, and concentrated. The residue was eluted with ethyl ace-tate/hexane (v/v10/90) through a silica gel column to give thecorresponding pure quinoxaline. Quinoxaline structures werecharacterized by 1H NMR, 13C NMR, FTIR, and GCeMS, and weremostly known compounds. For unknown compounds, elementalanalysis data were additionally obtained. 4.2.1. 2,3-Dimethylquinoxaline (5).19,31aef Yield 87%. Yellow solid,mp 109e110 C (lit.31f mp 105 C). TLC (10% ethyl acetate/hexane) Rf0.30; 1H NMR (400 MHz, CDCl3) d 7.91 (dd, 2H, J6.3, 3.5 Hz), 7.59(dd, 1H, J6.3, 3.5 Hz), 2.66 (s, 6H); 13C NMR (100 MHz, CDCl3)d 153.4, 141.0, 128.7, 128.2, 23.1; IR (KBr) 3109, 3078, 3030, 2995,2953, 2914, 1490, 1398, 1164 cm1;GCeMS m/z (rel intensity) 158(M, 99), 117 (100), 76 (28), 50 (12).
References:
Go, Ahra;Lee, Geunsoo;Kim, Jaeho;Bae, Seolhee;Lee, Byung Min;Kim, Byeong Hyo [Tetrahedron,2015,vol. 71,# 8,p. 1215 - 1226]