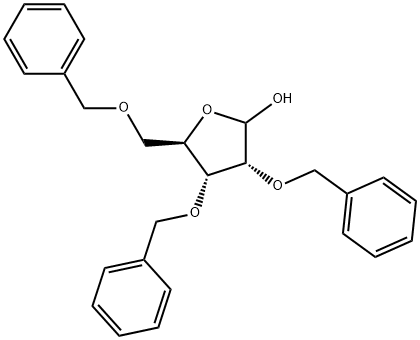
2,3,5-TRI-O-BENZYL-L-ARABINOFURANOSE synthesis
- Product Name:2,3,5-TRI-O-BENZYL-L-ARABINOFURANOSE
- CAS Number:16838-89-4
- Molecular formula:C26H28O5
- Molecular Weight:420.5
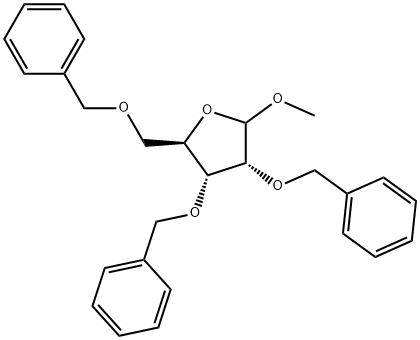
64363-77-5
56 suppliers
$38.00/100mg

16838-89-4
33 suppliers
inquiry
Yield: 86%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;water for 3 h;Reflux;
Steps:
1.1 Preparation of (3S,5R)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)tetrahydrofuran-2-ol
12 g (73.1mmol) of ribose was weighed and added to 250mL eggplant bottle, 160 mL of analytically pure DMF was added and 17g NaH was added in ice bath and 38 mL BnBr was dropped slowly. The temperature was slowly raised to room temperature, and reacted for 12 hours. After completion of the reaction by TLC, methanol was added slowly to extract the reaction and again extracted with EA, washed with water, and the crude product was chromatographed (PE: EA = 15: 1 → 9: 1) to give the compound (2R,4S)-3,4-bis(benzyloxy)-2-((benzyloxy)methyl)-5-methoxytetrahydrofuran (28.6 g, 65.8 mmol, 90%). The compound 38a (3.5 g, 8.0 mmol) was weighed into a 100 mL eggplant flask, 28 mL of 1,4-dioxane was added to dissolve it, and 28 mL of 4N HCl was added. The temperature was refluxed for 3 hours. After completion of the reaction tracked by TLC, the reaction mixture was extracted with EA, washed with water, and then washed with saturated NaHCO3 solution and saturated NaCl solution. The crude product was chromatographed under reduced pressure (PE: EA = 5: 1 → 3.5: 1) to obtain (3S,5R)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)tetrahydrofuran-2-ol (2.9 g,6.9 mmol, 86%).
References:
Jiangxi Normal University;Liao, Jinxi;Hu, Shijin;Xiong, Bin;Sun, Jiansong CN106167475, 2016, A Location in patent:Paragraph 0021-0023; 0025
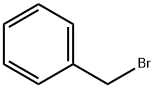
100-39-0
434 suppliers
$10.00/10 g

16838-89-4
33 suppliers
inquiry
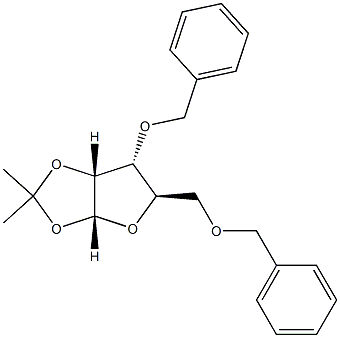
55735-86-9
8 suppliers
inquiry

16838-89-4
33 suppliers
inquiry
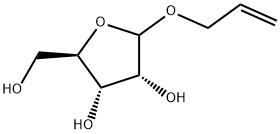
541546-94-5
0 suppliers
inquiry

16838-89-4
33 suppliers
inquiry
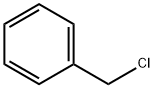
100-44-7
646 suppliers
$13.50/250G

16838-89-4
33 suppliers
inquiry
