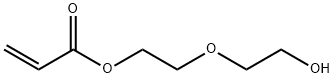
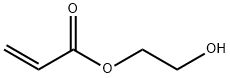
2-(2-hydroxyethoxy)ethyl acrylate synthesis
- Product Name:2-(2-hydroxyethoxy)ethyl acrylate
- CAS Number:13533-05-6
- Molecular formula:C7H12O4
- Molecular Weight:160.17
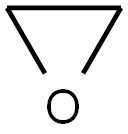
75-21-8
233 suppliers
$33.29/crm44609
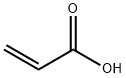
79-10-7
701 suppliers
$20.16/100 mL
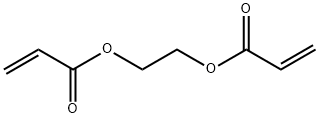
2274-11-5
76 suppliers
$66.90/100mg

13533-05-6
11 suppliers
inquiry
818-61-1
481 suppliers
$6.00/25g
Yield:-
Reaction Conditions:
with 10H-phenothiazine;chromium(II) acetate at 60; under 7500.75 Torr; for 6.5 h;
Steps:
1 Example !
An SUS-316-made autoclave of 1 L in capacity with a stirrer was charged with 282 g of ethylene oxide, 2.10 g of chromium acetate (as a catalyst), and 0.42 g of phenothiazine (as a polymerization inhibitor), and then internal air of the autoclave was replaced with nitrogen gas, and then the temperature was raised to 60 C and the internal pressure was adjusted to 1.0 MPa. Next, 420 g of acrylic acid was supplied at an almost constant rate over a period of 2 hours (during this supply, the molar ratio of ethylene oxide/acrylic acid was set at not less than 1.1 and, after the end of the supply, this ratio was 1.1), while 60 C was kept to carry out a reaction. After the supply of the acrylic acid had been completed, the reaction temperature was kept constant at 60 C to continue the reaction till the amount of the unreacted acrylic acid (measured by neutralization titration) decreased to 0.10 weight %. As a result of the continuation of the reaction for 4.5 hours, the unreacted acrylic acid decreased to 0.10 weight %, so the reaction liquid was cooled. A gas-chromatographic analysis of the resultant reaction liquid showed a hydroxyethyl acrylate (objective product) concentration of 91 weight %, an ethylene glycol diacrylate (diester) concentration of 0.09 weight %, and a diethylene glycol monoacrylate (diaddition product) concentration of 7.2 weight %. Next, the resultant reaction liquid was transferred into a glass round-bottom flask of 1 L in capacity, as set to a vacuum distillation apparatus, to carry out purification by distillation under a vacuum of 2 to 10 hPa in the internal temperature range of 60 to 100 C while the reaction liquid was caused to bubble with air at 10 mL/min, thus obtaining 626 g of hydroxyethyl acrylate.A gas-chromatographic analysis of the resultant hydroxyethyl acrylate showed its purity of 97.0 weight % and an ethylene glycol diacrylate (impurity) content of 0.10 weight %, and an analysis by neutralization titration showed an acid component content of 0.06 weight %.
References:
Nippon Shokubai Co., Ltd. EP1371629, 2003, A1 Location in patent:Page 9
814-68-6
386 suppliers
$21.21/5gm:

111-46-6
1081 suppliers
$5.00/25g

13533-05-6
11 suppliers
inquiry

75-21-8
233 suppliers
$33.29/crm44609

79-10-7
701 suppliers
$20.16/100 mL

13533-05-6
11 suppliers
inquiry

818-61-1
481 suppliers
$6.00/25g