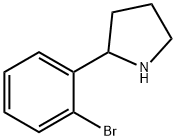
2-(2-Bromophenyl)pyrrolidine synthesis
- Product Name: 2-(2-Bromophenyl)pyrrolidine
- CAS Number:129540-24-5
- Molecular formula:C10H12BrN
- Molecular Weight:226.11
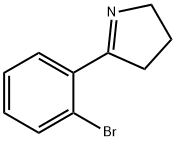
129540-26-7
1 suppliers
inquiry

129540-24-5
85 suppliers
$29.00/100mg
Yield:-
Reaction Conditions:
Stage #1: 2-(2-bromophenyl)-1-pyrrolinewith sodium tetrahydroborate;acetic acid in methanol at -65 - 20;
Stage #2: with hydrogenchloride in water;
Stage #3: with water;sodium hydroxide
Steps:
10
Compound 10 2(2-Bromophenyl)pyrrolidine 5-(2-Bromophenyl)-3,4-dihydro-2H-pyrrole (compound 8; 393 mmol, 88 g) was dissolved in methanol (1300 mL), then the acetic acid (330 mL) was added and the solution cooled to -65° C. under a nitrogen atmosphere. Sodium borohydride (589 mmol, 22.28 g) was added portionwise over 1 hour. The reaction was stirred at -65° C. for 30 minutes, then the cooling bath was removed and the reaction mixture temperature was allowed to rise to room temperature. The bulk of the methanol was removed under vacuum then 5N HCl (950 mL) was added and the solution extracted with ether (2×500 mL). The aqueous solution was then basified with sodium hydroxide pellets (310 g) with ice-bath cooling, maintaining the reaction temperature less than 30° C. The basified aqueous was then extracted with ethyl acetate (3×800 mL), the combined organics washed with brine (800 mL), dried with sodium sulfate, evaporated and chromatographed on 1 Kg of silica gel eluting with 19:1 to 9:1 methylene chloride-ethanol to give 2-(2-bromophenyl)pyrrolidine (68.5 g).
References:
US2010/113493,2010,A1 Location in patent:Page/Page column 8
6091-64-1
176 suppliers
$10.00/1g

129540-24-5
85 suppliers
$29.00/100mg