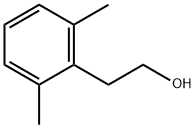
2-(2,6-DIMETHYLPHENYL)ETHANOL synthesis
- Product Name:2-(2,6-DIMETHYLPHENYL)ETHANOL
- CAS Number:30595-80-3
- Molecular formula:C10H14O
- Molecular Weight:150.22
Yield: 67.3%
Reaction Conditions:
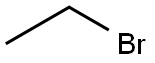
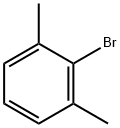
Stage #1:ethyl bromide;2-Bromo-m-xylene with magnesium in tetrahydrofuran at 30; for 7 h;Inert atmosphere;
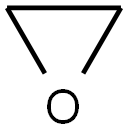
Stage #2: with oxirane at 30;Cooling with ice;Inert atmosphere;
Steps:
1 Example 1 2-(2,6-dimethylphenyl)ethanol
20 mL of dry tetrahydrofuran and 7.13 g (297 mmol) of magnesium powder were added to a 250 mL three-necked flask, then the reflux apparatus was removed to fill the reaction flask with tetrahydrofuran vapor. Drain the tetrahydrofuran vapor with a water pump until it is drained. Stop depressurization, pass nitrogen, install the dropping funnel, and cooled to 30 °C. 40 mL of dry tetrahydrofuran and 2.01 mL (27 mmol) of Bromoethane were added to the dropping funnel, and rapidly flowed into the reaction flask under stirring and nitrogen protection. Further, 50 g (270 mmol) of 2,6-dimethylbromobenzene and 80 mL of dry tetrahydrofuran were added to the dropping funnel, and the mixture was dropped into a reaction flask under stirring at 30 ° C under a nitrogen atmosphere, and the mixture was dropped over 1 h. After the completion of the dropwise addition, stirring was continued at 30 ° C for 6.5 h, and there was almost no remaining magnesium powder, and the reaction liquid was grayish black. Cooled to room temperature. 16.4 mL (324 mmol) of ethylene oxide was extracted with a syringe, and the mixture was poured into a reaction solution while stirring in an ice salt bath, and the mixture was stirred for 30 minutes under ice-cold bath, and stirred at 30 ° C under nitrogen atmosphere overnight, and the reaction mixture was unchanged. 100 mL of a saturated ammonium chloride solution was added dropwise to the reaction solution while stirring in an ice salt bath, and the layers were separated, the upper layer was a yellow liquid, and the lower layer was a gray viscous solid. The upper liquid was decanted and rotary evaporated to dryness to obtain a yellow oil. The lower solid was dissolved in 100 mL of dilute hydrochloric acid (1:1 by volume), extracted with dichloromethane (100 mL×3), and the above yellow oil was dissolved with dichloromethane, the combined dichloromethane solution (yellow liquid) was washed with a saturated sodium chloride solution and dried over anhydrous magnesium sulfate. After suction filtration, the filtrate was rotary evaporated to dryness to obtain a yellow liquid, that is crude compound 2 product. The crude product was purified by silica gel column chromatography (eluent: petroleum ether: ethyl acetate mass ratio = 5:1) to give a white solid, 27.38, yield 67.3%.TLC: Rf = 0.62 (petroleum ether: ethyl acetate mass ratio = 5:1).
References:
Nanjing Medical University;Li Tingyou;Ma Rui·T·shimisi;Qin Yajuan;Shi Saijian;Xu Jian CN108503579, 2018, A Location in patent:Paragraph 0033; 0034
75-21-8
239 suppliers
$33.29/crm44609
576-22-7
329 suppliers
$10.00/1g

30595-80-3
13 suppliers
inquiry

75-21-8
239 suppliers
$33.29/crm44609

576-22-7
329 suppliers
$10.00/1g

30595-80-3
13 suppliers
inquiry
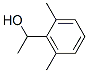
19447-06-4
15 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

30595-80-3
13 suppliers
inquiry
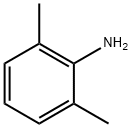
87-62-7
463 suppliers
$10.00/5 g

30595-80-3
13 suppliers
inquiry