2,2,2-Trichloroethyl chloroformate synthesis
1synthesis methods
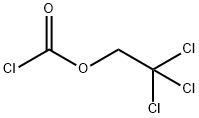
- Product Name:2,2,2-Trichloroethyl chloroformate
- CAS Number:17341-93-4
- Molecular formula:C3H2Cl4O2
- Molecular Weight:211.86
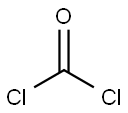
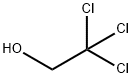
2,2,2-Trichloroethyl chloroformate is widely available commercially. It can be readily prepared by passing Phosgene into a solution of 2,2,2- Trichloroethanol in benzene/diethylaniline or benzene/ether/pyridine. An alternate two-step approach (93% yield) has been described that employs S-Ethyl Chlorothioformate instead of phosgene.
Yield:-
Reaction Conditions:
with pyridine in dichloromethane
Steps:
3 EXAMPLE 3
EXAMPLE 3 79 g (1.0 mol) of pyridine in 50 ml of CH2 Cl2 are added, at -20° to -5° C, to 149 g (1.0 mol) of 2,2,2-trichloroethanol and 70 ml (1.05 mols) of condensed phosgene in 400 ml of CH2 Cl2 and the mixture is stirred for a further 1 hour at 20° C. It is washed at 0° C with water, 10% strength sulphuric acid and water and dried (Na2 SO4) and the solvent is stripped off. After vacuum distillation, 177 g (85%) of 2,2,2-trichloroethoxycarbonyl chloride are obtained. Boiling point10 = 65°-67° C.
References:
Bayer Aktiengesellschaft US4111933, 1978, A