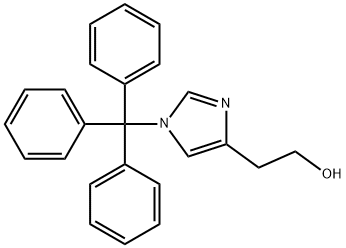
2-(1-trityl-1H-iMidazol-4-yl)ethanol synthesis
- Product Name:2-(1-trityl-1H-iMidazol-4-yl)ethanol
- CAS Number:127607-62-9
- Molecular formula:C24H22N2O
- Molecular Weight:354.44
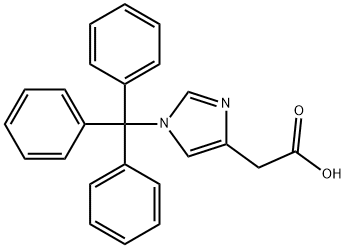
168632-03-9
16 suppliers
inquiry

127607-62-9
18 suppliers
inquiry
Yield:127607-62-9 87%
Reaction Conditions:
with diborane in tetrahydrofuran at 0 - 25;
Steps:
1.2 EXAMPLE 1
[001461 2-(1-trityl-1H-imidazol-4-yl)ethanol: 2-(1-Trityl-1H-imidazol-4-yl) acetic acid (42 g, 114.00 mmol, 1.0 equiv) was suspended in THF (420 mL) and cooled to 0 °C. To this was added BH3 (1M in THF, 228.28 mL, 2.0 equiv). The clear solution obtained was stirred at 0 °C for 60 mm, then warmed to room temperature until LCMS indicated completion of the reaction. The solution was cooled again to 0 °C and quenched carefully with water (300 mL). The resulting solution was extracted with ethyl acetate (3 x 100 mL) and the organic layers combined and dried over anhydrous Na2504 and evaporated to give a sticky residue which was taken up in ethanolamine (800 mL) and heated to 90 °C for 2 h. The reaction was transferred to a separatory funnel, diluted with EtOAc (1 L) and washed with water (3 x 600 mL). The organic phase was dried over anhydrous Na2504 and evaporated afford 35 g (87%) of 2-[1-(triphenylmethyl)-1H-imidazol-4-ylj ethanol as a white solid, which was used in the next step without further purification. LCMS (ESI): m/z = 355.1 [M+Hjt
References:
WO2016/109361,2016,A2 Location in patent:Paragraph 00146
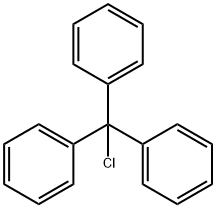
76-83-5
708 suppliers
$6.00/25g

127607-62-9
18 suppliers
inquiry
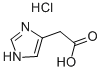
3251-69-2
213 suppliers
$15.00/250mg

127607-62-9
18 suppliers
inquiry