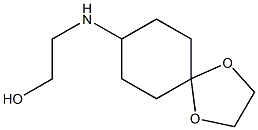
2-{1,4-dioxaspiro[4.5]decan-8-ylamino}ethan-1-ol synthesis
- Product Name:2-{1,4-dioxaspiro[4.5]decan-8-ylamino}ethan-1-ol
- CAS Number:862730-43-6
- Molecular formula:C10H19NO3
- Molecular Weight:201.2628
Yield:-
Reaction Conditions:
Stage #1:cyclohexanedione monoethylene ketal;ethanolamine with sodium tris(acetoxy)borohydride in dichloromethane at 20; for 18 h;
Stage #2: with sodium hydroxide;water in dichloromethane
Steps:
13.1
EXAMPLE 13 3-[4-({(1R)-1-(4-chlorobenzyl)-2-[4-cyclohexyl-4-(1H-1,2,4-triazol-1-ylmethyl)piperidin-1-yl-2-oxoethyl}amino)cyclohexyl]-1,3-oxazolidin-2-one hydrochloride (compound No. 103) 13.1: 2-(1,4-dioxaspiro[4.5]dec-8-ylamino)-ethanol 3.12 g of 1,4-dioxaspiro[4.5]decan-8-one are dissolved in 80 ml of dichloromethane in the presence of 1.16 g of ethanolamine. 6.75 g of sodium triacetoxyborohydride are then added under N2. Stirring is maintained at ambient temperature for 18 h. After hydrolysis with a 1N aqueous sodium hydroxide solution, extraction is carried out with dichloromethane until the aqueous phase is completely depleted. After drying over MgSO4 and concentration to dryness, 4.0 g of 2-(1,4-dioxaspiro[4.5]dec-8-ylamino)ethanol are obtained, which product is subsequently used as it is. 13.2: 3-(1,4-dioxaspiro[4.5]dec-8-yl)-1,3-oxazolidin-2-one
References:
SANOFI-AVENTIS US2007/149562, 2007, A1 Location in patent:Page/Page column 33
![1,4-Dioxaspiro[4.5]decan-8-one](/CAS/GIF/4746-97-8.gif)
