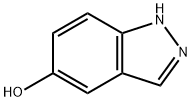
1H-Indazol-5-ol synthesis
- Product Name:1H-Indazol-5-ol
- CAS Number:15579-15-4
- Molecular formula:C7H6N2O
- Molecular Weight:134.14
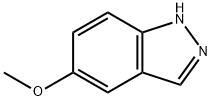
94444-96-9
134 suppliers
$24.00/250mg

15579-15-4
209 suppliers
$5.00/250mg
Yield: 71%
Reaction Conditions:
with boron tribromide in dichloromethane at 20; for 10 h;
Steps:
4.b Reference Example 4; Synthesis of 1H-indazol-5-ol; (b) Synthesis of 1H-indazol-5-ol
A methylene chloride solution of boron tribromide (18.5 ml, 18.5 mmol) was added to a solution of 5-methoxy-1H-indazole (1.24 g, 8.40 mmol) in methylene chloride (84 ml) at 0°C and stirred at room temperature for 10 hours. Then, water was poured into the reaction solution in an ice-water bath, followed by extraction with ethyl acetate. The organic layer was washed with a saturated aqueous sodium hydrogencarbonate solution and then a saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, and distilled under reduced pressure to remove the solvent, and the resulting residue was purified by a silica gel column chromatography (eluent: chloroform/methanol = 96/4) to obtain 1H-indazol-5-ol (877 mg, 71%).1H-NMR (DMSO-d6) δ; 6.88 (1H, dd, J=8.8, 2.2Hz), 6.96 (1H, d, J=2.2Hz), 7.34 (1H, d, J=8.8Hz), 7.84 (1H, s).
References:
Sumitomo Pharmaceuticals Company, Limited EP1403255, 2004, A1 Location in patent:Page 44
19335-11-6
316 suppliers
$10.00/1 g

15579-15-4
209 suppliers
$5.00/250mg
543-87-3
68 suppliers
$20.00/1g
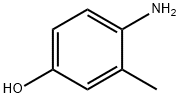
2835-99-6
374 suppliers
$8.00/10g
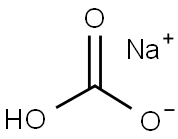
144-55-8
1356 suppliers
$10.00/25g

15579-15-4
209 suppliers
$5.00/250mg
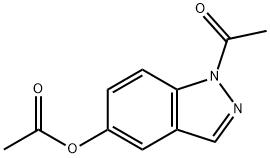
36174-07-9
11 suppliers
inquiry

15579-15-4
209 suppliers
$5.00/250mg

2835-99-6
374 suppliers
$8.00/10g

15579-15-4
209 suppliers
$5.00/250mg