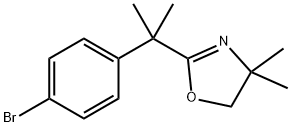
2-[1-(4-BROMOPHENYL)-1-METHYLETHYL]-4,4-DIMETHYL-4,5-DIHYDROOXAZOLINE synthesis
- Product Name:2-[1-(4-BROMOPHENYL)-1-METHYLETHYL]-4,4-DIMETHYL-4,5-DIHYDROOXAZOLINE
- CAS Number:192775-97-6
- Molecular formula:C14H18BrNO
- Molecular Weight:296.2
Yield: 4.61 g (0.0156 mmole, 84%)
Reaction Conditions:
with CH3I;potassium hexamethylsilazane;acetic acid in dichloromethane;water;isopropyl alcohol
Steps:
2 Preparation of 4-bromo-α,α-dimethyl-α-(4,4-dimethyloxazolin-2-yl) toluene
Example 2 Preparation of 4-bromo-α,α-dimethyl-α-(4,4-dimethyloxazolin-2-yl) toluene A 250 mL three neck round bottomed flask was charged with 5.0 g (0.0186 mole) of 4-bromo-α-(4,4-dimethyloxazolin-2-yl)toluene, prepared according to Example 1, and 50 mL of dry THF under N2. KHMDS, 27 mL (0.0279 mole, 1.5 eq), was then slowly added over 10 minutes. A color change to deep orange was observed. After stirring the mixture for 15 minutes at room temperature, 1.16 mL (0.0186 mole, 1 equiv.) of methyl iodide was added in one portion. The reaction exothermed to 46° C., and white solid precipitated while the solution retained a pale yellow tint. After stirring for 1 hour, another 27 mL (0.0279 mole, 1.5 equiv.) of KHMDS was added causing the temperature of the reaction to rise from 27° to 30° C. and the color to change to orange. The reaction was stirred for an additional 20 minutes and, thereafter, a second equivalent of CH3I was added. An aliquot was removed, quenched with water, and extracted with ethyl acetate. TLC analysis (4:1 hexane/ethyl acetate) showed the presence of the more polar 4-bromo-α-methyl-α-(4,4-dimethyloxazolin-2-yl) toluene ("mono adduct"). An additional 0.2 mL of CH3I was added which turned the pale yellow solution to white. The reaction mixture was then added to 100 mL 10% acetic acid/water along with 250 mL methylene chloride. The organic layer was washed twice with 50 mL brine and dried with sodium sulfate. After concentration and drying at room temperature and a pressure of 0.1 mm Hg overnight, 5.65 g (103%) of a yellowish solid was obtained. The solid was dissolved in 30 mL isopropanol and 20 mL of water was slowly added until an oil had formed. To the mixture, 5 mL of isopropanol was added with heating to dissolve all of the oil. The oil crystallized upon cooling in an ice bath, yielding 4.61 g (0.0156 mmole, 84%) of pure 4-bromo-α,α-dimethyl-α-(4,4-dimethyloxazolin-2-yl) toluene no trace of mono adduct.
References:
Albany Molecular Research, Inc. US6201124, 2001, B1
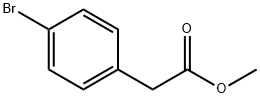
41841-16-1
213 suppliers
$5.00/5g
![2-[1-(4-BROMOPHENYL)-1-METHYLETHYL]-4,4-DIMETHYL-4,5-DIHYDROOXAZOLINE](/CAS/GIF/192775-97-6.gif)
192775-97-6
57 suppliers
inquiry
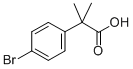
32454-35-6
212 suppliers
$48.00/1g
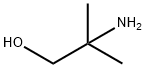
124-68-5
539 suppliers
$4.76/25g
![2-[1-(4-BROMOPHENYL)-1-METHYLETHYL]-4,4-DIMETHYL-4,5-DIHYDROOXAZOLINE](/CAS/GIF/192775-97-6.gif)
192775-97-6
57 suppliers
inquiry
![2-[(4-Bromophenyl)methyl]-4,5-dihydro-4,4-dimethyloxazole](/CAS/20210111/GIF/176324-85-9.gif)
