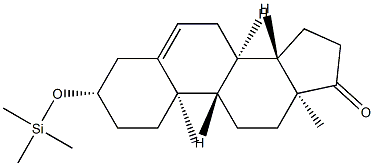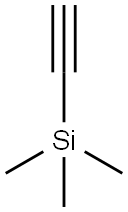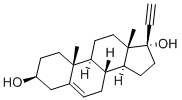
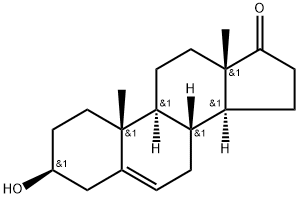
17A-PREGN-5-EN-20-YNE-3B,17-DIOL synthesis
- Product Name:17A-PREGN-5-EN-20-YNE-3B,17-DIOL
- CAS Number:3604-60-2
- Molecular formula:C21H30O2
- Molecular Weight:314.46
Yield:3604-60-2 87%
Reaction Conditions:
Stage #1: trimethylsilylacetylenewith n-butyllithium in tetrahydrofuran at 0;Inert atmosphere;
Stage #2: PdCl2(PPh3)2 in tetrahydrofuran at 20;
Steps:
2
?-Butyl lithium is added slowly to Me3Si-C≡CH in THF under a nitrogen atmosphere at approximately 0 °C to produce the lithium acetylide Me3Si-C≡C-Li. The temperature was raised to about 20° C, and TMS-DHEA was added as a solution in THF, and stirred for about 3 hours. The reaction was quenched by raising the temperature to about 40° C, followed by the slow addition of methanol. Liberated acetylene is purged under slight vacuum. Concentrated KOH was then slowly added until gas evolution subsides, and the volume was reduced by approximately 50% by vacuum distillation at approximately 45 °C. Excess 6 N HCI was slowly added, while maintaining the temperature at approximately 40° C. The reaction mixture was diluted with water and chilled to approximately 5° C before collecting the product by filtration and washing the filter cake with cold 50/50 methanol water. The product was dried with warm nitrogen to an LOD of not more than to 0.5% to provide approximately 87% isolated yield of the title compound.
References:
WO2009/149392,2009,A1 Location in patent:Page/Page column 13; 14

60-29-7
0 suppliers
$31.19/50g
53-43-0
607 suppliers
$27.00/10g
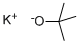
865-47-4
704 suppliers
$14.00/25g

74-86-2
76 suppliers
inquiry
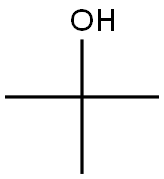
75-65-0
770 suppliers
$10.00/10ml

3604-60-2
12 suppliers
inquiry