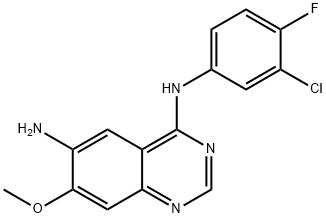
N-(3-chloro-4-fluorophenyl)-7-Methoxy-6-aminoquinazolin-4-aMine synthesis
- Product Name:N-(3-chloro-4-fluorophenyl)-7-Methoxy-6-aminoquinazolin-4-aMine
- CAS Number:179552-75-1
- Molecular formula:C15H12ClFN4O
- Molecular Weight:318.73
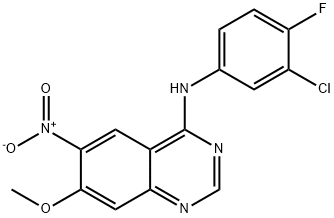
179552-74-0
118 suppliers
inquiry

179552-75-1
96 suppliers
$73.34/1g
Yield: 99%
Reaction Conditions:
with hydrogen;nickel in tetrahydrofuran
Steps:
10
(3-Chloro-4-fluoro-phenyl)-(7-fluoro-6-nitro-quinazolin-4-yl)-amine was suspended in 100 ml methanol and 2 ml 50% NaOH in water was added and the mixture was heated at 70° C. for 2 hours. The mixture was then poured into water and stirred vigorously for 30 minutes, then filtered and washed with water and dried under vacuum at 60° C. overnight to give 7.2 g of (3-Chloro-4-fluoro-phenyl)-(7-methoxy-6-nitro-quinazolin-4-yl)-amine. 7.1 g of (3-Chloro-4-fluoro-phenyl)-(7-methoxy-6-nitro-quinazolin-4-yl)-amine was reduced using Raney nickel catalyst in THF, then filtered and evaporated to give 6.4 g N4-(3-Chloro-4-fluoro-phenyl)-7-methoxy-quinazoline-4,6-diamine (99% yield). This product was reacted with 4-Chloro-but-2-enoyl chloride as described in Scheme 1 to provide 4-Chloro-but-2-enoic acid [4-(3-chloro-4-fluoro-phenylamino)-7-methoxy-quinazolin-6-yl]-amide. 300 g of 4-Chloro-but-2-enoic acid [4-(3-chloro4-fluoro-phenylamino)-7-methoxy-quinazolin-6-yl]-amide and 78 mg azepane were dissolved in 5 ml THF and purged with nitrogen. 0.25 ml DIEA was added and the mixture was stirred at 70° C. for 2 days. The mixture was then diluted with 20 ml ethyl acetate, washed with water and brine and dried over Na2SO4. The resulting solids were flash chromatographed with 0-4% methanol in chloroform. The product was dissolved in CH2Cl2 and treated with excess HCl and ether, then evaporated to dryness to give 115 mg of 4-Azepan-1-yl-but-2-enoic acid [4-(3-chloro-4-fluoro-phenylamino)-7-methoxy-quinazolin-6-yl]-amide (33% yield). (M+H)+ @484.
References:
Pfizer Inc US2005/250761, 2005, A1 Location in patent:Page/Page column 22
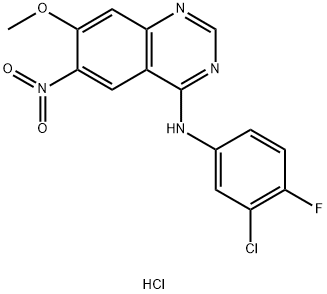
1012057-48-5
0 suppliers
inquiry

179552-75-1
96 suppliers
$73.34/1g
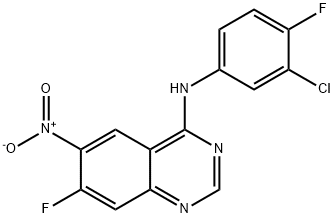
162012-67-1
337 suppliers
$8.00/1g

179552-75-1
96 suppliers
$73.34/1g
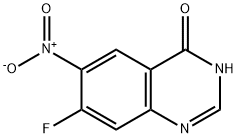
162012-69-3
382 suppliers
$6.00/250mg

179552-75-1
96 suppliers
$73.34/1g
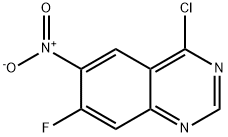
162012-70-6
156 suppliers
$125.00/25mg

179552-75-1
96 suppliers
$73.34/1g
