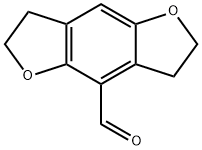
4-Formyl-2,3,6,7-Tetrahydrobenzo[1,2-B:4,5-B']Difuran synthesis
- Product Name:4-Formyl-2,3,6,7-Tetrahydrobenzo[1,2-B:4,5-B']Difuran
- CAS Number:178557-13-6
- Molecular formula:C11H10O3
- Molecular Weight:190.2
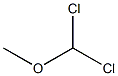
4885-02-3
194 suppliers
$10.00/1g
![2,3,6,7-Tetrahydro-benzo[1,2-b:4,5-b']difuran](/CAS/GIF/81926-24-1.gif)
81926-24-1
77 suppliers
$22.00/250mg
![4-Formyl-2,3,6,7-Tetrahydrobenzo[1,2-B:4,5-B']Difuran](/CAS/GIF/178557-13-6.gif)
178557-13-6
8 suppliers
inquiry
Yield:178557-13-6 82%
Reaction Conditions:
Stage #1: 4,10-dioxatricyclo[7.3.0.03,7]dodeca-1,3(7),8-trienewith tin(IV) chloride in dichloromethane; for 0.0833333 h;Inert atmosphere;
Stage #2: Dichloromethyl methyl ether in dichloromethane;Inert atmosphere;
Steps:
2.4 Synthesis of compound 5
A solution of 3.47 g (21.2 mmol) of the 2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b']difuran 4 in 80 mL of dry CH2Cl2 was stirred under N2 and cooled over an ice bath. Tin (IV) chloride (3.25 mL, 27.81 mmol) was added to the solution, and the mixture was stirred for 5 min. Dichloromethyl methyl ether (2.13 mL, 23.53 mol) in 5 mL of CH2Cl2 was then introduced into the mixture dropwise over a 5 min period. After the mixture had stirred for 30 min, the reaction was quenched by the addition of 50 mL of ice-H2O, and the layers were separated. The aqueous phase was extracted with 2 × 30 mL of CH2Cl2, and the organic fractions were combined. The organic extract was washed with 3 N HCl (3×80 mL), 80 mL of H2O, and 80 mL of brine, dried over Na2SO4 and evaporated to furnish the crude product, which was chromatographed on silica gel (petroleum ether 60-90 °C : ethyl acetate = 12:1) to afford the products 5 (3.30 g, 82%) as yellow crystals. Yield 82%. Yellow solid. 1H NMR (300 MHz, CDCl3) δ: 10.27 (1H, s), 6.87 (1H, s), 4.71-4.55 (4H, m), 3.45 (2H, t, J = 8.84 Hz),3.17 (2H, t, J = 9.2 Hz). 13C NMR (75 MHz, CDCl3) δ : 189.07 (CH), 157.51 (C), 154.64 (C), 128.35 (C), 125.13 (C), 116.02 (C), 112.67 (CH), 72.79 (CH2), 72.34 (CH2), 30.44 (CH2), 29.24 (CH2) .
References:
Zhang, Chao-Bo;Liu, Yang;Liu, Zheng-Fen;Duan, Sheng-Zu;Li, Min-Yan;Chen, Wen;Li, Yan;Zhang, Hong-Bin;Yang, Xiao-Dong [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 8,p. 1808 - 1814] Location in patent:supporting information
37142-37-3
30 suppliers
$112.00/100mg
![4-Formyl-2,3,6,7-Tetrahydrobenzo[1,2-B:4,5-B']Difuran](/CAS/GIF/178557-13-6.gif)
178557-13-6
8 suppliers
inquiry

178557-12-5
22 suppliers
$110.50/50mg
![4-Formyl-2,3,6,7-Tetrahydrobenzo[1,2-B:4,5-B']Difuran](/CAS/GIF/178557-13-6.gif)
178557-13-6
8 suppliers
inquiry
123-31-9
849 suppliers
$13.00/25g
![4-Formyl-2,3,6,7-Tetrahydrobenzo[1,2-B:4,5-B']Difuran](/CAS/GIF/178557-13-6.gif)
178557-13-6
8 suppliers
inquiry