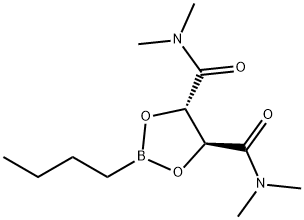
2-BUTYL-1,3,2-DIOXABOROLANE-4S,5S-DICARBOXYLIC ACID BIS(DIMETHYLAMIDE) synthesis
- Product Name:2-BUTYL-1,3,2-DIOXABOROLANE-4S,5S-DICARBOXYLIC ACID BIS(DIMETHYLAMIDE)
- CAS Number:161344-84-9
- Molecular formula:C12H23BN2O4
- Molecular Weight:270.13
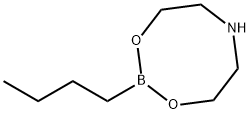
92527-13-4
18 suppliers
inquiry
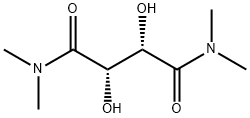
63126-52-3
87 suppliers
$9.00/1g

161344-84-9
58 suppliers
$17.00/100mg
Yield:-
Reaction Conditions:
in dichloromethane;water at 20; for 1 h;
Steps:
26.B
Example 26B; 2-Butyl-f 1.3.21dioxaborolane-(S.S)-4.5-dicarboxylic acid bis-dimethylamide; 2-(But-1-yl)-tetrahydro-4H-1,3,6,2-dioxazaborocine [CAS 92527-13-4] was prepared from n-butylboronic acid and 2-(2-hydroxy-ethylamino)-ethanol [CAS 111-42-2] as reported in Organic Synthesis, 1998, 76, 86-96. This dioxazaborocine (3 g, 17.5 mmol) and (2S,3S)-2,3-dihydroxy-N,N,N',N'- tetramethyl-butanediamide [CAS 63126-52-3] (4.65 g) were dissolved in anhydrous dichloromethane (95 ml.) under N2. Brine (30 ml_) was added. The resulting mixture was stirred at room temperature for 1 hour. The two layers were separated, and the aqueous layer was extracted with dichloromethane (30 ml_). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated in vacuo to provide the title compound as an oil. 1H NMR (300 MHz, CDCI3): δ 0.82-0.9 (m, 5H), 1.25-1.45 (m, 4H), 2.98 (s, 6H), 3.2 (s, 6H), 5.52 (S, 2H). MS (DCI-NH3) m/z 271 (M+H)+.
References:
ABBOTT LABORATORIES WO2007/150010, 2007, A2 Location in patent:Page/Page column 75
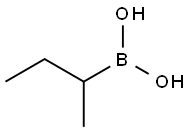
4426-47-5
346 suppliers
$6.00/1g

63126-52-3
87 suppliers
$9.00/1g

161344-84-9
58 suppliers
$17.00/100mg
