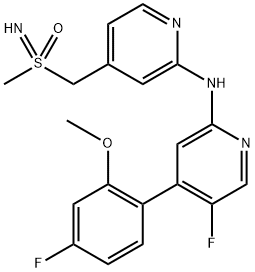
(rac)-5-fluoro-4-(4-fluoro-2-methoxyphenyl)-N-{4-[(S-methylsulfonimidoyl)methyl]pyridin-2-yl}pyridin-2-amine synthesis
- Product Name:(rac)-5-fluoro-4-(4-fluoro-2-methoxyphenyl)-N-{4-[(S-methylsulfonimidoyl)methyl]pyridin-2-yl}pyridin-2-amine
- CAS Number:1610358-59-2
- Molecular formula:C19H18F2N4O2S
- Molecular Weight:404.44
![Acetamide, 2,2,2-trifluoro-N-[[[2-[[5-fluoro-4-(4-fluoro-2-methoxyphenyl)-2-pyridinyl]amino]-4-pyridinyl]methyl]methyl-λ4-sulfanylidene]-](/CAS/20211123/GIF/1610359-06-2.gif)
1610359-06-2
1 suppliers
inquiry
![(rac)-5-fluoro-4-(4-fluoro-2-methoxyphenyl)-N-{4-[(S-methylsulfonimidoyl)methyl]pyridin-2-yl}pyridin-2-amine](/CAS/20211123/GIF/1610358-59-2.gif)
1610358-59-2
20 suppliers
inquiry
Yield:1610358-59-2 50 mg
Reaction Conditions:
with potassium permanganate in acetone at 50; for 8 h;Reagent/catalyst;Solvent;
Steps:
1 Preparation of end product:
Under argon, a solution of 2,2,2-trifluoroacetamide (195 mg; 1.73 mmol) in dioxane (0.5 mL) was added dropwise to a solution of sodium tert. -butoxide (111 mg; 1.15 mmol) in dioxane (0.6 mL), so that the temperature of the mixture remained below 10 °C. Subsequently, a freshly prepared solution of 1,3- dibromo-5,5-dimethylhydantoin (247 mg; 0.86 mmol) in dioxane (0.6 mL) / THF (1.0 mL) was added dropwise to the stirred mixture, so that the temperature of the mixture remained below 10°C. Then the mixture was stirred for 10 minutes at 10 °C. Finally, a solution of 5-fluoro-4-(4-fluoro-2- methoxyphenyl)-Ar-{4-[(methylsulfanyl)methyl]pyridin-2-yl}pyridin-2-amine (430 mg; 1.15 mmol) in dioxane (1.0 mL) was added dropwise to the stirred mixture, so that the temperature of the mixture remained below 10 °C. The mixture was stirred for 60 minutes at 10 °C. The batch was diluted with toluene (2.0 mL) under cooling and an aqueous solution of sodium sulfite (145 mg; 1.15 mmol in 2.0 mL water) was added so that the temperature of the mixture remained below 15 °C. An aqueous solution of sodium chloride was added and the batch was extracted with ethyl acetate (3x). The combined organic phases were filtered using a Whatman filter and concentrated to give crude 2,2,2-trifluoro-Ar-{ [(2-{ [5- fluoro-4-(4-fluoro-2-methoxyphenyl)pyridin-2-yl]amino }pyridin-4-yl)methyl](methyl)-λ4- sulfanylidenejacetamide, that was used without further purification. Acetone (6.0 mL) and potassium permanganate (814 mg; 5.15 mmol) were added to the residue and the mixture was stirred at 50 °C for 90 minutes. Additional potassium permanganate (223 mg; 1.42 mmol) was added and stirring was continued at 50 °C for 4 hours. Finally, additional potassium permanganate (305 mg; 1.93 mmol) was added and stirring was continued at 50 °C for 150 minutes. After cooling, the batch was filtered, the residue was washed with acetone and the combined filtrates were concentrated. The residue was dissolved in MeOH (60 mL), potassium carbonate (182 mg; 1.32 mmol) was added and the reaction mixture was stirred for 20 minutes at RT. The batch was diluted with an aqueous solution of sodium chloride and extracted with DCM (3x). The combined organic phases were filtered using a Whatman filter and concentrated. The residue was purified by preparative HPLC to give the desired product (50 mg; 0.12 mmol). NMR (400MHz, d6-DMSO, 300K) δ = 9.80 (s, 1H), 8.20 (m, 1H), 8.16 (m, 1H), 7.79 (m, 1H), 7.59 (m, 1H), 7.34 (m, 1H), 7.09 (m, 1H), 6.91 (m, 2H), 4.36 (m, 2H), 3.80 (s, 3H), 3.72 (s, 1H), 2.88 (s, 3H).
References:
WO2014/76091,2014,A1 Location in patent:Page/Page column 99-100
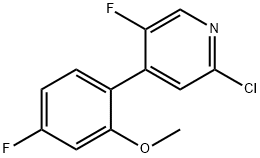
1602486-03-2
4 suppliers
inquiry
![(rac)-5-fluoro-4-(4-fluoro-2-methoxyphenyl)-N-{4-[(S-methylsulfonimidoyl)methyl]pyridin-2-yl}pyridin-2-amine](/CAS/20211123/GIF/1610358-59-2.gif)
1610358-59-2
20 suppliers
inquiry
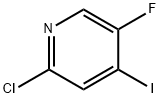
884494-49-9
164 suppliers
$6.00/100mg
![(rac)-5-fluoro-4-(4-fluoro-2-methoxyphenyl)-N-{4-[(S-methylsulfonimidoyl)methyl]pyridin-2-yl}pyridin-2-amine](/CAS/20211123/GIF/1610358-59-2.gif)
1610358-59-2
20 suppliers
inquiry
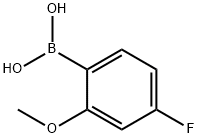
179899-07-1
205 suppliers
$9.00/250mg
![(rac)-5-fluoro-4-(4-fluoro-2-methoxyphenyl)-N-{4-[(S-methylsulfonimidoyl)methyl]pyridin-2-yl}pyridin-2-amine](/CAS/20211123/GIF/1610358-59-2.gif)
1610358-59-2
20 suppliers
inquiry
![2-Pyridinamine, 5-fluoro-4-(4-fluoro-2-methoxyphenyl)-N-[4-[(methylthio)methyl]-2-pyridinyl]-](/CAS/20210305/GIF/1602486-04-3.gif)
1602486-04-3
1 suppliers
inquiry
![(rac)-5-fluoro-4-(4-fluoro-2-methoxyphenyl)-N-{4-[(S-methylsulfonimidoyl)methyl]pyridin-2-yl}pyridin-2-amine](/CAS/20211123/GIF/1610358-59-2.gif)
1610358-59-2
20 suppliers
inquiry
