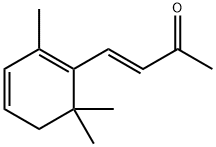
(E)-4-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)but-3-en-2-one synthesis
- Product Name:(E)-4-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)but-3-en-2-one
- CAS Number:14398-35-7
- Molecular formula:C13H18O
- Molecular Weight:190.28
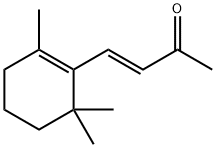
79-77-6
249 suppliers
$10.00/25g

14398-35-7
2 suppliers
inquiry
Yield:14398-35-7 70%
Reaction Conditions:
Stage #1: (E)-β-iononewith N-Bromosuccinimide in toluene; for 1 h;Irradiation;
Stage #2: with sodium carbonate in N,N-dimethyl-formamide;toluene at 20; for 0.5 h;
Steps:
(3E)-4-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)but-3-en-2-one (3)
A solution of β-ionone (2) (2.1137 g, 11 mmol) and N-bromosuccinimide (2.3085 g, 13 mmol) in 100 mL of anhydrous toluene (carbon tetrachloride can also be used as solvent) was irradiated with a 100 W tungsten lamp for 1 hour. [1] After cooling to room temperature and filtering, anhydrous sodium carbonate (2.5111 g, 24 mmol) and dimethylformamide (25 mL) were added to the filtrate, and the reaction mixture was stirred for 30 minutes. The solvent was distilled off and the resulting residue was extracted with ethyl ether (200 mL). The organic layer was washed with 5% aqueous HCl (100 mL) and saturated brine, and dried over anhydrous MgSO4. After solvent removal under reduced pressure, the residue was purified by column chromatography on silica gel using n-hexane:ethyl acetate (8:2) as eluent to give compound 3 (1.4640 g, 7.7 mmol, 70% yield) as a yellow liquid. The NMR characterization data are identical to the literature values. [1] 1H NMR (CDCl3, 500 MHz): 7.27 (d, J = 16.4 Hz, 1H), 6.21 (d, J = 16.4 Hz, 1H), 5.88 (m, 2H), 2.31 (s, 3H), 2.11 (d, J = 2.1 Hz, 2H), 1.91 (s, 3H), 1.08 (s, 6H). 13C NMR (CDCl3, 75 MHz): 198.0, 141.5, 135.7, 132.5, 130.1, 129.4, 128.0, 39.8, 33.8, 27.1, 26.3, 26.3, 20.1.
References:
Lazaro, Aline S.;Ribeiro, Pedro H. Zana;Sairre, Mirela I.;Donate, Paulo M. [Synthetic Communications,2015,vol. 45,# 11,p. 1374 - 1378] Location in patent:supporting information
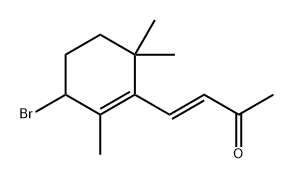
84817-80-1
0 suppliers
inquiry

14398-35-7
2 suppliers
inquiry
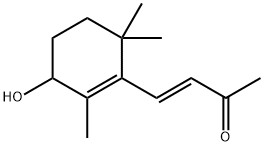
14398-34-6
7 suppliers
inquiry

14398-35-7
2 suppliers
inquiry
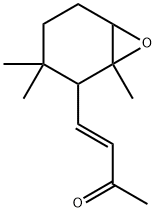
190059-33-7
17 suppliers
$50.82/1g

14398-35-7
2 suppliers
inquiry
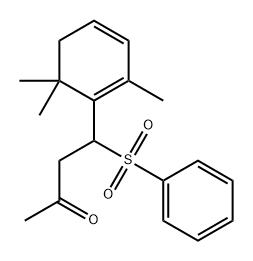
66408-59-1
0 suppliers
inquiry

14398-35-7
2 suppliers
inquiry