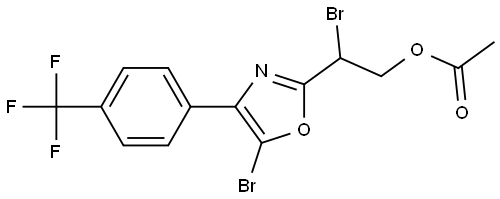
2-Oxazoleethanol, β,5-dibromo-4-[4-(trifluoromethyl)phenyl]-, 2-acetate synthesis
- Product Name:2-Oxazoleethanol, β,5-dibromo-4-[4-(trifluoromethyl)phenyl]-, 2-acetate
- CAS Number:1403883-99-7
- Molecular formula:C14H10Br2F3NO3
- Molecular Weight:457.04
![2-Oxazoleethanol, 5-bromo-4-[4-(trifluoromethyl)phenyl]-, 2-acetate](/CAS/20240320/GIF/1403883-98-6.gif)
1403883-98-6
0 suppliers
inquiry
![2-Oxazoleethanol, β,5-dibromo-4-[4-(trifluoromethyl)phenyl]-, 2-acetate](/CAS/20240320/GIF/1403883-99-7.gif)
1403883-99-7
0 suppliers
inquiry
Yield:1403883-99-7 39%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethane at 80;
Steps:
2.5
To a solution of 2-(5-bromo-4-(4-(trifluoromethyl)phenyl)oxazol-2-yl)ethyl acetate (0.325 g, 0.86 mmol), in CC14 (15 mL) was added NBS (0.153 g, 0.86 mmol) and AIBN (0.015 g, 0.09 mmol). The resulting reaction mixture was stirred at 80°C for 5-6 h. After the completion of reaction, the mixture was filtered and the filtrate concentrated in vacuo. The residue was purified by flash chromatography (1.0% EtOAc/hexane) to yield 2-bromo-2-(5-bromo-4-(4-(trifluoromethyl)phenyl)oxazol-2- yl)ethyl acetate (0.155 g, 39%).
References:
WO2012/142671,2012,A1 Location in patent:Page/Page column 31-33
383-53-9
131 suppliers
$5.00/1g
![2-Oxazoleethanol, β,5-dibromo-4-[4-(trifluoromethyl)phenyl]-, 2-acetate](/CAS/20240320/GIF/1403883-99-7.gif)
1403883-99-7
0 suppliers
inquiry
![2-Oxazoleacetic acid, 4-[4-(trifluoromethyl)phenyl]-, ethyl ester](/CAS/20210111/GIF/1403883-96-4.gif)
1403883-96-4
0 suppliers
inquiry
![2-Oxazoleethanol, β,5-dibromo-4-[4-(trifluoromethyl)phenyl]-, 2-acetate](/CAS/20240320/GIF/1403883-99-7.gif)
1403883-99-7
0 suppliers
inquiry