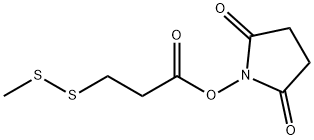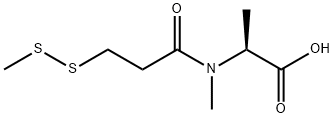
(S)-2-(N-Methyl-3-(Methyldisulfanyl)propanaMido)propanoic acid synthesis
- Product Name:(S)-2-(N-Methyl-3-(Methyldisulfanyl)propanaMido)propanoic acid
- CAS Number:138148-62-6
- Molecular formula:C8H15NO3S2
- Molecular Weight:237.34
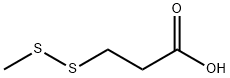
3913-67-5
305 suppliers
$6.00/1g
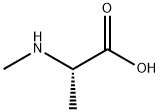
138148-59-1
17 suppliers
inquiry
543-27-1
268 suppliers
$10.00/5g

138148-62-6
31 suppliers
inquiry
Yield: 0.556 g (34%)
Reaction Conditions:
with triethylamine in tetrahydrofuran;water
Steps:
1 Synthesis of Maytansinoid Derivatives
N-methyl-N-(3-methyldithio-propanoyl)-L-alanine (5b). To a stirred solution of 3-methyldithiopropanoic acid (3b) (1.00 g, 6.57 mmol) in dry THF (20 ml) at -10° C. under argon was added isobutylchloroformate (0.897 g, 6.57 mmol) and triethylamine (0.665 g, 6.57 mmol) and the reaction mixture was stirred for 15 minutes. A mixture of N-methyl-L-alanine (0.677 g, 6.57 mmol) and triethylamine (1.33 g, 13.14 mmol) in water (10 ml) was added and the reaction mixture was stirred at room temperature overnight. The mixture was then diluted with water (50 ml), acidified (aqueous HCl), and extracted with ethyl acetate (4*50 ml). The combined organic layers were dried over sodium sulfate, the solvent evaporated under reduced pressure and the residue chromatographed over silica gel eluding with methylene chloride/ethyl acetate to yield 0.556 g (34%) white crystals: mp 98°-100° C. 1 H NMR (CDCl3) δ1.3 (3H, d), 2.2 (3H, s), 2.7 (4H, m), 4.5 (1H, q), 10.7 (1H, s). Anal. Calculated for C8 H15 NO3 S2: C, 40.49; H, 6.37; N, 5.90; mol wt. 237.33. Found: C, 40.42; H, 6.41; N, 5.93.
References:
ImmunoGen Inc. US5208020, 1993, A