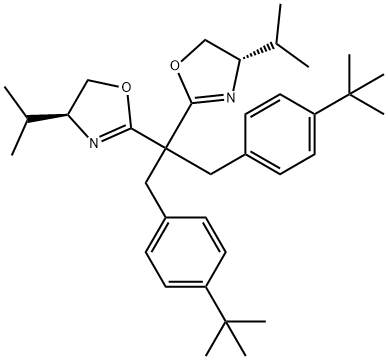
(4S,4'S)-2,2'-[2-[4-(1,1-dimethylethyl)phenyl]-1- [[4-(1,1- dimethylethyl)phenyl]methyl]ethylidene]bis[4,5- dihydro-4-(1-methylethyl)-Oxazole synthesis
- Product Name:(4S,4'S)-2,2'-[2-[4-(1,1-dimethylethyl)phenyl]-1- [[4-(1,1- dimethylethyl)phenyl]methyl]ethylidene]bis[4,5- dihydro-4-(1-methylethyl)-Oxazole
- CAS Number:1373357-05-1
- Molecular formula:C35H50N2O2
- Molecular Weight:530.78
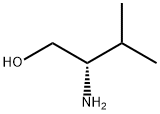
2026-48-4
432 suppliers
$11.60/1mL
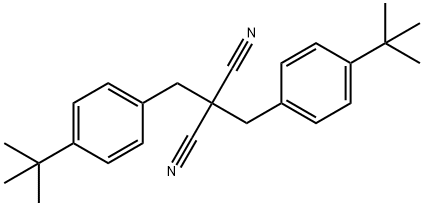
1373356-95-6
1 suppliers
inquiry
![(4S,4'S)-2,2'-[2-[4-(1,1-dimethylethyl)phenyl]-1-
[[4-(1,1-
dimethylethyl)phenyl]methyl]ethylidene]bis[4,5-
dihydro-4-(1-methylethyl)-Oxazole](/CAS/20200515/GIF/1373357-05-1.gif)
1373357-05-1
15 suppliers
inquiry
Yield:1373357-05-1 40%
Reaction Conditions:
with zinc(II) chloride in 1,2-dichloro-benzene at 150; for 12 h;Inert atmosphere;
Steps:
4.3. Preparation of bisoxazoline ligands 1f-q
General procedure: To a solution of dialkylated malononitrile in o-dichlorobenzene (30 mL per gram of dialkylated malononitrile) were added zinc chloride (3.0 equiv) and l-valinol (3.0 equiv), and the reaction mixture was stirred at 150 °C for 12 h. After the reaction was complete, the reaction mixture was cooled to room temperature. To the reaction mixture were added water (3 mL per gram of dialkylated malononitrile) and ethylenediamine (6 mL per gram of dialkylated malononitrile), and the reaction mixture was stirred at room temperature for 1 h. Water (30 mL per gram of dialkylated malononitrile) and dichloromethane (30 mL per gram of dialkylated malononitrile) was added to the reaction mixture and the aqueous layer was extracted with dichloromethane (30 mL per gram of dialkylated malononitrile). The combined organic layer was washed with brine, dried over MgSO4, and evaporated. The residue was purified by silica gel chromatography to afford the bisoxazoline ligand.
References:
Sawada, Takashi;Nakada, Masahisa [Tetrahedron Asymmetry,2012,vol. 23,# 5,p. 350 - 356] Location in patent:experimental part
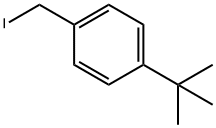
140874-33-5
2 suppliers
inquiry
![(4S,4'S)-2,2'-[2-[4-(1,1-dimethylethyl)phenyl]-1-
[[4-(1,1-
dimethylethyl)phenyl]methyl]ethylidene]bis[4,5-
dihydro-4-(1-methylethyl)-Oxazole](/CAS/20200515/GIF/1373357-05-1.gif)
1373357-05-1
15 suppliers
inquiry