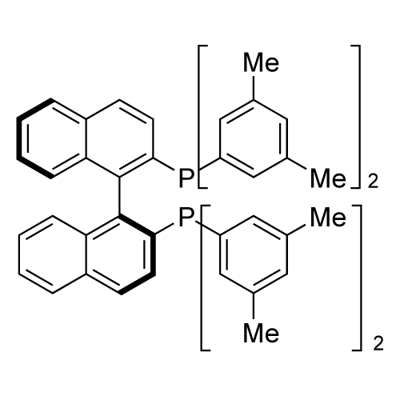
(S)-(-)-2,2'-Bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl synthesis
- Product Name:(S)-(-)-2,2'-Bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl
- CAS Number:135139-00-3
- Molecular formula:C52H48P2
- Molecular Weight:734.89
![Phosphine oxide, [1,1'-binaphthalene]-2,2'-diylbis[bis(3,5-dimethylphenyl)-, (+)- (9CI)](/CAS/20210305/GIF/137244-17-8.gif)
137244-17-8
0 suppliers
inquiry
![(S)-(-)-2,2'-Bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl](/CAS/GIF/135139-00-3.gif)
135139-00-3
136 suppliers
$25.00/100mg
Yield:-
Reaction Conditions:
with sodium hydroxide;trichlorosilane;triethylamine in 5,5-dimethyl-1,3-cyclohexadiene;
Steps:
5 Synthesis of (-)-2,2'-bis[di-(3,5-dimethylphenyl)phosphino]-1,1'-binaphthyl, (-)-(I-1)
EXAMPLE 5 Synthesis of (-)-2,2'-bis[di-(3,5-dimethylphenyl)phosphino]-1,1'-binaphthyl, (-)-(I-1) To 0.77 g (1.00 mmole) of the optically active compound (-)-(IX-1) as obtained in Example 4 were added 6.5 ml of xylene and 2.7 ml (19.37 mmole) of triethylamine. After the optically active compound was dissolved by stirring, 1.7 ml (16.87 mmole) of trichlorosilane was added dropwise thereto over a period of 20 to 30 minutes. The mixture was reacted at 100° C. for 1 hour, at 120° C. for 1 hour, and then at 145° C. for 4 hours. The reaction mixture was then cooled to room temperature, and 8.5 ml of xylene and 10 ml of a 30% sodium hydroxide solution were added thereto. The resulting mixture was stirred at 70° C. for 30 minutes, followed by liquid separation. The organic layer was washed with water and dried over magnesium sulfate, and the toluene was then removed by evaporation under reduced pressure to obtain 0.62 g of a reduction prtoduct. This product was resolved by silica gel column chromatography (hexane:ethyl acetate=8:1), thereby obtaining 0.24 g (yield 32.7%) of (-)-2,2'-bis[di-(3,5-dimethylphenyl)phosphino]-1,1'-binaphthyl (hereinafter referred to as "(-)-3,5-DMBINAP"). Further, the same procedures as the above were repeated except that the optically active compound, (+)-(IX-1), as obtained in Example 4 was used. Thus, 0.22 g (yield 30.0%) of (+)-2,2'-bis[di-(3,5-dimethylphenyl)phosphino]-1,1'-binaphthyl was obtained. [α]D25 for (+)-isomer: +163.3° (c=1, chloroform). [α]D25 for (-)-isomer: -163.7° (c=1, chloroform).
References:
US5223632,1993,A
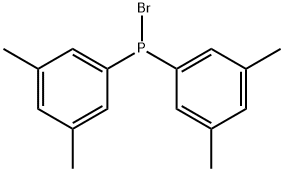
1342819-37-7
0 suppliers
inquiry
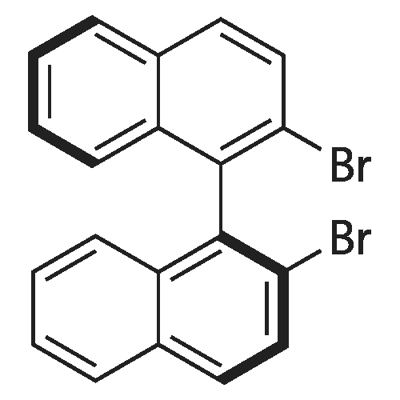
150024-49-0
29 suppliers
$80.00/50mg
![(S)-(-)-2,2'-Bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl](/CAS/GIF/135139-00-3.gif)
135139-00-3
136 suppliers
$25.00/100mg
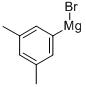
34696-73-6
49 suppliers
$226.00/50mL
![(S)-(-)-2,2'-Bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl](/CAS/GIF/135139-00-3.gif)
135139-00-3
136 suppliers
$25.00/100mg
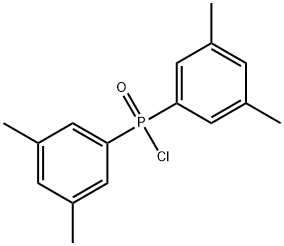
137219-83-1
1 suppliers
inquiry
![(S)-(-)-2,2'-Bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl](/CAS/GIF/135139-00-3.gif)
135139-00-3
136 suppliers
$25.00/100mg
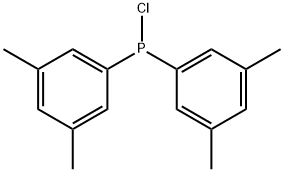
74289-57-9
79 suppliers
$47.00/250mg
![(S)-(-)-2,2'-Bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl](/CAS/GIF/135139-00-3.gif)
135139-00-3
136 suppliers
$25.00/100mg