2,3,4,5-TETRAHYDRO-8-METHOXY-1H-PYRIDO[4,3-B]INDOLE synthesis
- Product Name:2,3,4,5-TETRAHYDRO-8-METHOXY-1H-PYRIDO[4,3-B]INDOLE
- CAS Number:126912-70-7
- Molecular formula:C12H14N2O
- Molecular Weight:202.25
![tert-Butyl 8-methoxy-1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/GIF/1186099-85-3.gif)
1186099-85-3
15 suppliers
inquiry
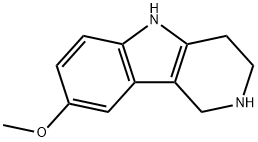
![2,3,4,5-TETRAHYDRO-8-METHOXY-1H-PYRIDO[4,3-B]INDOLE](/CAS/GIF/126912-70-7.gif)
126912-70-7
36 suppliers
$60.00/100mg
Yield:126912-70-7 100%
Reaction Conditions:
with hydrogenchloride in ethyl acetate at 20; for 1 h;
Steps:
12.2 Step 2) 8-methoxy-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole 12c
8-Methoxy-1,3,4,5-tetrahydropyrido[4,3-b]indole-2-carboxylic acid tert-butyl ester 12b (1.0 g, 3.3 mmol) was dissolved in ethyl acetate (1 mL ), add ethyl acetate solution of hydrogen chloride (8mL, 2mol/L), and react at room temperature for 1 hour. The reaction solution was concentrated, the resulting residue was dissolved in methanol (10 mL), sodium carbonate (0.70 g, 6.6 mmol) was added, and the reaction was carried out at room temperature for 1 hour. The reaction solution was filtered, and the filtrate was concentrated to obtain the title compound 12c (0.67 g, yield 100%) as a yellow solid.
References:
CN111196806,2020,A Location in patent:Paragraph 0559; 0564-0566
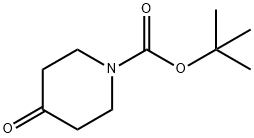
79099-07-3
0 suppliers
$5.00/5g
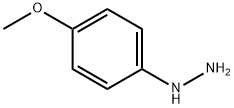
3471-32-7
36 suppliers
inquiry
![2,3,4,5-TETRAHYDRO-8-METHOXY-1H-PYRIDO[4,3-B]INDOLE](/CAS/GIF/126912-70-7.gif)
126912-70-7
36 suppliers
$60.00/100mg
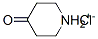
41979-39-9
0 suppliers
$15.00/10mg
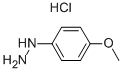
19501-58-7
401 suppliers
$7.00/250mg
![2,3,4,5-TETRAHYDRO-8-METHOXY-1H-PYRIDO[4,3-B]INDOLE](/CAS/GIF/126912-70-7.gif)
126912-70-7
36 suppliers
$60.00/100mg
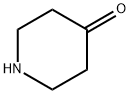
41661-47-6
0 suppliers
$45.00/250mg

19501-58-7
401 suppliers
$7.00/250mg
![2,3,4,5-TETRAHYDRO-8-METHOXY-1H-PYRIDO[4,3-B]INDOLE](/CAS/GIF/126912-70-7.gif)
126912-70-7
36 suppliers
$60.00/100mg

79099-07-3
0 suppliers
$5.00/5g
![2,3,4,5-TETRAHYDRO-8-METHOXY-1H-PYRIDO[4,3-B]INDOLE](/CAS/GIF/126912-70-7.gif)
126912-70-7
36 suppliers
$60.00/100mg