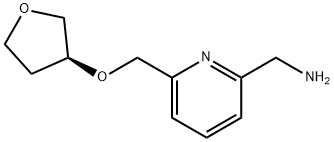
(S)-(6-((tetrahydrofuran-3-yloxy)methyl)pyridin-2-yl)methanamine synthesis
- Product Name:(S)-(6-((tetrahydrofuran-3-yloxy)methyl)pyridin-2-yl)methanamine
- CAS Number:1202402-79-6
- Molecular formula:C11H16N2O2
- Molecular Weight:208.26
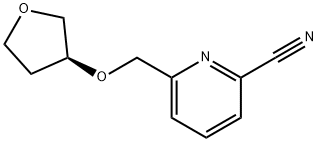
1202402-51-4
1 suppliers
inquiry

1202402-79-6
17 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: (S)-6-(((tetrahydrofuran-3-yl)oxy)methyl)-2-cyanopyridinewith borane-THF in tetrahydrofuran at 0;Inert atmosphere;Reflux;
Stage #2: with hydrogenchloride;water pH=1;
Stage #3: with sodium hydroxide in water; pH=7 - 14;
Steps:
1B
(S)-6-(Tetrahvdrofuran-3-yloKvmethylV2-pyridm&mi&thanamirte(S)-6-(Tetrahydrofuran-3-yloxymethyl)pyridine-2-carbonitrile (0.206 mol, 1 eq) was added to borane (1-M in THF, 290 mL, 0.290 mol, 3 eq) at 0 0C under nitrogen. The reaction was then heated to reflux for 5 hours, cooled to room temperature and quenched with 100 mL methanol (exothermic) and evaporated. 2 M aqueous HCl (310 mL) was added (pH 1) to the combined residues. After the quench, the mixture was brought to pH 7 with 5 M NaOH (35 mL). The aqueous mixture was extracted with 2 x 500 mL dichloromethane. These organic extracts were discarded. The aqueous phase was brought to pH 14 with 5-N NaOH (100 mL) and extracted with 2 x 1000 mL DCM. The combined organic extracts (from alkaline solution) were washed with 100 mL saturated brine, dried over Na2SO4 and evaporated to give the title compound (50.70 g, 39% over 2 steps) as an amber oil; LC-MS Retention time 0.62 min; (M+H)+ 205.
References:
WO2009/156737,2009,A1 Location in patent:Page/Page column 33
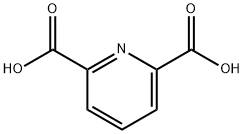
499-83-2
625 suppliers
$6.00/10g

1202402-79-6
17 suppliers
inquiry

5453-67-8
289 suppliers
$6.00/5g

1202402-79-6
17 suppliers
inquiry
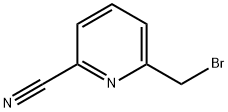
104508-24-9
57 suppliers
inquiry

1202402-79-6
17 suppliers
inquiry
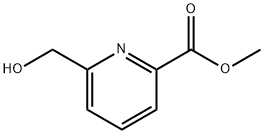
39977-44-1
153 suppliers
$13.00/250mg

1202402-79-6
17 suppliers
inquiry