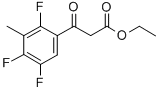
ETHYL 3-(2,4,5-TRIFLUORO-3-METHYLPHENYL)-3-OXOPROPANOATE synthesis
- Product Name:ETHYL 3-(2,4,5-TRIFLUORO-3-METHYLPHENYL)-3-OXOPROPANOATE
- CAS Number:112822-88-5
- Molecular formula:C12H11F3O3
- Molecular Weight:260.21
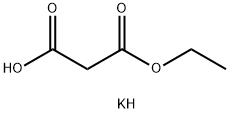
6148-64-7
397 suppliers
$5.00/10g
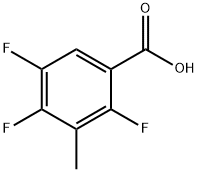
112822-85-2
125 suppliers
$34.00/250mg

112822-88-5
17 suppliers
$503.08/5MG
Yield:112822-88-5 3.9 g
Reaction Conditions:
Stage #1: ethyl potassium malonatewith hydrogenchloride in water;Cooling;
Stage #2: with triethylamine;magnesium chloride in acetonitrile at 20; for 4 h;Cooling with ice;
Stage #3: 3-methyl-2,4,5-trifluorobenzoic acidFurther stages;
Steps:
12 Reference Example 12 (0459) Ethyl 3-(4-fluoro-2-(hydroxymethyl)phenyl]amino-2-(3-methyl-2,4,5-trifluorobenzoyl)acrylate
14.5 g of potassium monoethyl malonate was added to 20 mL of 6 mol/L cold hydrochloric acid and then the mixture was extracted with diethyl ether. The extract was washed with saturated brine and then dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure and then 20 mL of acetonitrile, 1.9 g of magnesium chloride, and 4.1 g of triethylamine were added to the resulting residue under ice cooling. The mixture was stirred at room temperature for 4 hours to obtain a suspension. 15 mL of acetonitrile and 2.8 g of carbonyldiimidazole were added to 3.5 g of 3-methyl-2,4,5-trifluorobenzoic acid and the mixture was stirred at room temperature for 4 hours. The reaction solution was added to the aforementioned suspension at room temperature and the mixture was stirred at 80° C. for 3 hours. 100 mL of 1 mol/L hydrochloric acid was added and then the mixture was extracted with ethyl acetate. The extract was dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure and the residue was subjected to silica gel column chromatography (n-hexane/ethyl acetate=200) to obtain 3.9 g of ethyl 2-(3-methyl-2,4,5-trifluoro)benzoylacetate. 3 mL of the acetic anhydride and 3 mL of ethyl orthoformate were added to the obtained compound and the mixture was stirred at 130° C. for 6 hours. The solvent was evaporated under reduced pressure. The residue was subjected to azeotropic distillation twice with toluene and the resulting residue was dissolved in dichloromethane to prepare a 1 mol/L solution for use in the following reaction. 29.7 mL of N,N-dimethylformamide and 29.7 mL of the dichloromethane solution of ethoxyacrylate prepared earlier were added to 4.19 g of 2-amino-5-fluorophenylmethanol and the mixture was stirred at room temperature for 1 hour. The solvent was evaporated under reduced pressure and ethyl acetate was added to the residue. The mixture was washed with water. The organic layer was dried using anhydrous sodium sulfate and the solvent was evaporated under reduced pressure. The resulting residue was dispersed in hexane, collected by filtration, and dried under reduced pressure to obtain 10.4 g of the title compound. 1H-NMR (DMSO-d6): δ 0.91 (0.9H, t, J=7.1 Hz), 1.00 (2.1H, t, J=7.2 Hz), 2.21 (3H, brs), 3.97-4.03 (2H, m), 4.60 (2H, d, J=4.6 Hz), 5. 79-5.83 (1H, m), 7.20-7.29 (2H, m), 7.36-7.45 (1H, m), 7.58 (0.3H, dd, J=4.5, 8.6 Hz), 7.65 (0.7H, dd, J=4.8, 8.7 Hz), 8.36 (0.3H, d, J=14.3 Hz), 8.46 (0.7H, d, J=13.8 Hz).
References:
US2019/276407,2019,A1 Location in patent:Paragraph 0459-0463

112822-85-2
125 suppliers
$34.00/250mg
105-53-3
763 suppliers
$5.00/25g

112822-88-5
17 suppliers
$503.08/5MG
79-37-8
472 suppliers
$17.67/10gm:

112822-85-2
125 suppliers
$34.00/250mg

105-53-3
763 suppliers
$5.00/25g

112822-88-5
17 suppliers
$503.08/5MG