10Z-HYMENIALDISINE synthesis
- Product Name:10Z-HYMENIALDISINE
- CAS Number:82005-12-7
- Molecular formula:C11H10BrN5O2
- Molecular Weight:324.13
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

82005-12-7
49 suppliers
$159.00/500μg
Yield: 81%
Reaction Conditions:
with hydrogenchloride in methanol
Steps:
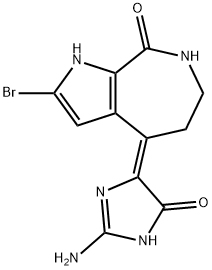
10 Synthesis of 4-(2-amino-4-oxy-2-imidazolin-5-ylidene)-2-bromo-4,5,6,7-tetrahydropyrrolo[2,3-c]azepin-8-one (hymenialdisine) hydrochloride (20)
Example 10 Synthesis of 4-(2-amino-4-oxy-2-imidazolin-5-ylidene)-2-bromo-4,5,6,7-tetrahydropyrrolo[2,3-c]azepin-8-one (hymenialdisine) hydrochloride (20) A 3 ml amount of 10% hydrochloric acid was added to a 3 ml methanol solution of 300 mg of the compound (18) synthesised in Example 8. The mixture was stirred at 90° C. for 1 hour. The solvent was distilled off under reduced pressure to obtain a residue which was then purified by silica gel column chromatography (chloroform:methanol: 2% acetic acid=65:35:10). The solvent was distilled off under reduced pressure, then the residue was treated by 1 ml of isopropyl alcohol saturated with hydrochloric acid to give the crude crystals which were recrystallized by methanol/ether to obtain 150 mg of the above-referenced compound (20) (yield 81%).
References:
Suntory Limited US5621099, 1997, A
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

82005-12-7
49 suppliers
$159.00/500μg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

125118-55-0
12 suppliers
inquiry

82005-12-7
49 suppliers
$159.00/500μg
![β-Alanine, N-[(5-bromo-1H-pyrrol-2-yl)carbonyl]-](/CAS/20210305/GIF/872971-60-3.gif)
872971-60-3
0 suppliers
inquiry

82005-12-7
49 suppliers
$159.00/500μg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

82005-12-7
49 suppliers
$159.00/500μg