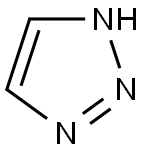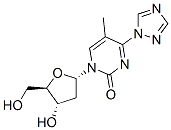
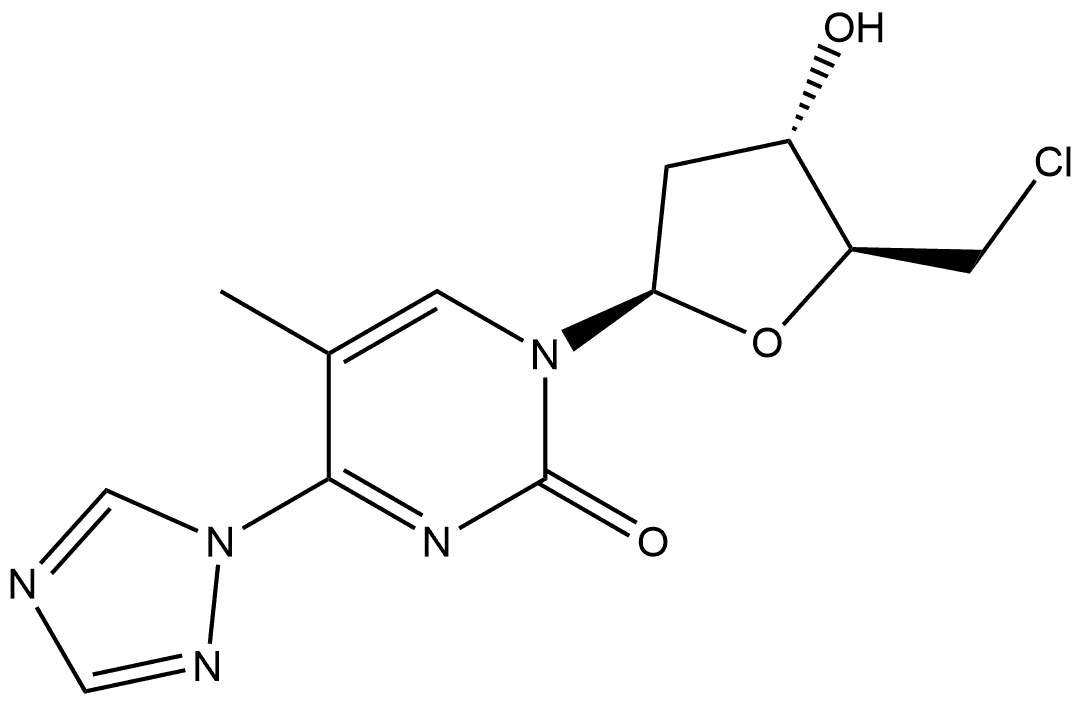
2(1H)-Pyrimidinone, 1-(2-deoxy-b-D-erythro-pentofuranosyl)-5-methyl-4-(1H-1,2,4-triazol-1-yl) synthesis
- Product Name:2(1H)-Pyrimidinone, 1-(2-deoxy-b-D-erythro-pentofuranosyl)-5-methyl-4-(1H-1,2,4-triazol-1-yl)
- CAS Number:109389-25-5
- Molecular formula:C12H15N5O4
- Molecular Weight:293.28
Yield:109389-25-5 88%
Reaction Conditions:
Stage #1: thymidinewith chloro-trimethyl-silane;triethylamine in acetonitrile at 20; for 1.5 h;
Stage #2: 1,2,3-triazolewith trichlorophosphate in acetonitrile at 0; for 5 h;
Stage #3: with acetic acid in methanol at 20; for 4.5 h;
Steps:
4.2.28. 1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methyl-4-(1H-1,2,4-triazol-1-yl)pyrimidin-2(1H)-one (34)[14]
Triethylamine (10.5mL, 75.3mmol) and chlorotrimethylsilane (3.2mL, 25.2mmol) were added to a stirred suspension of thymidine4(1.22g, 5.04mmol) in acetonitrile (40mL) at room temperature. After 1.5h, the reaction mixture was cooled (ice water bath), and 1,2,4-1H-triazole (3.12g, 45.2mmol) and phosphorus oxychloride (0.95mL, 10.2mmol) were added with continued stirring. After a period of 5h, the product was poured into saturated aqueous sodium hydrogen carbonate (250mL), and the resulting mixture was extracted with dichloromethane (2×25mL). The combined organic extracts were dried (Na2SO4) and evaporated under reduced pressure. Acetic acid-methanol (1:4 v/v, 15mL) was added to the residue, and the resulting solution was allowed to stir at room temperature. After 4.5h, diethyl ether (30mL) was added dropwise, with stirring, to this solution over a period of 30min. After a further period of 2h, colourless crystals of the target compound34(1.30g, 88%) were collected by filtration.1H NMR (300MHz, DMSO):δ9.23 (s, 1H), 8.62 (s, 1H), 8.38 (s, 1H), 6.13 (t, 1H,J=6Hz), 5.31 (bs, 1H), 5.22 (bs, 1H), 4.28-4.25 (m, 1H), 3.92-3.60 (m, 3H), 3.12-3.05 (m, 1H), 2.42-2.11 (m, 5H);13C NMR (75MHz, CDCl3)δ: 157.9, 153.5, 153.2, 148.0, 145.4, 104.5, 88.2, 87.0, 69.4, 60.5, 45.7, 41.1, 16.3, 8.7; HRMS (EI+): m/z for [C12H16N5O4]+calcd. 294.1197; found 294.1201.
References:
Saudi, Milind;Zmurko, Joanna;Kaptein, Suzanne;Rozenski, Jef;Neyts, Johan;Van Aerschot, Arthur [European Journal of Medicinal Chemistry,2014,vol. 76,p. 98 - 109]
50-89-5
649 suppliers
$9.00/1g

109389-25-5
7 suppliers
inquiry
10457-18-8
0 suppliers
inquiry

109389-25-5
7 suppliers
inquiry
288-88-0
820 suppliers
$10.00/5g

50-89-5
649 suppliers
$9.00/1g
111160-28-2
0 suppliers
inquiry

109389-25-5
7 suppliers
inquiry