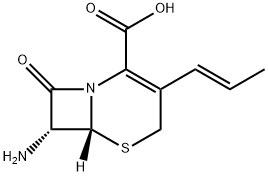
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-aMino-8-oxo-3-(1E)-1-propenyl-, (6R,7R)- synthesis
- Product Name:5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-aMino-8-oxo-3-(1E)-1-propenyl-, (6R,7R)-
- CAS Number:107937-01-9
- Molecular formula:C10H12N2O3S
- Molecular Weight:240.28
![5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 8-oxo-7-[(2-phenylacetyl)amino]-3-(1-propen-1-yl)-, (4-methoxyphenyl)methyl ester, (6R,7R)-](/CAS/20200611/GIF/190790-85-3.gif)
190790-85-3
0 suppliers
inquiry
![7-AMINO-3-[(Z)-PROP-1-ENYL]-3-CEPHEM-4-CARBOXYLIC ACID](/CAS/GIF/106447-44-3.gif)
106447-44-3
56 suppliers
$145.00/5mg
![5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-aMino-8-oxo-3-(1E)-1-propenyl-, (6R,7R)-](/CAS/20180808/GIF/107937-01-9.gif)
107937-01-9
34 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: with pyridine;phosphorus pentachloride in dichloromethane at 20; for 0.5 h;
Stage #2: (7R,8R)-7-phenylacetamido-3-[propen-1-yl]-3-cephem-4-carboxylic acid p-methoxybenzyl ester in dichloromethane; for 2 h;
Stage #3: with propylene glycol;water;ortho-cresolmore than 3 stages;
Steps:
5
22.8 g of phosphorus pentachloride, 150 ml of methylenechloride, and 8.88 ml of pyridine were placed in a 20 °C reactor and stirred for 30 minutes. 30g (62. 6mmol) of the 7-phenylacetamido-3-[propen-1-yl]-3-cephem-4-carboxylic acid p-methoxybenzyl ester prepared in Example 2 was dropwise added thereto and stirred for 2 hours. The reaction mixture was cooled to-10°C and was stirred for 2 hours after addition of 30 ml of 1, 2-propanediol and then for 2 hours after addition of 120 ml of cresol. 200 ml of distilled water was dropwise added thereto and stirred for 1 hour. After phase separation, an aqueous layer was sent to a crystallization bath, and an organic layer was extracted with 300 ml of 2N HCI and then the extract was sent to the crystallization bath. 200 ml of a 30% sodium hydroxide solution was dropwise added to the crystallization bath for crystallization and then cooled to 0°C. An obtained solid was filtered and dried in vacuum to give 12g (50mol, yield 80%, Z/E=10. 1/1) of the titled compound as a beige solid. H-NMR (8, D20+NaHC03) : 1.69 and 1.88 (3H, each, d, 6. 0Hz,-CH=CH-CH3), 3.38 and 3.72 (2H, Abq, 17Hz, H-2), 5.18 (1H, d, 5. 0Hz, H-6), 5.51 (1H, d, H-7), 5.8 (1H, m,-CH=CH-CH3), 6.06 (1 H, d, 11 Hz,-CH=CH-CH3)
References:
WO2005/42543,2005,A1 Location in patent:Page/Page column 18