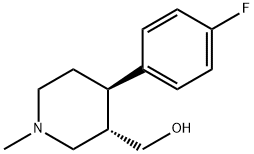
(3S,4R)-4-(4-Fluorophenyl)-3-hydroxymethyl-1-methylpiperidine synthesis
- Product Name:(3S,4R)-4-(4-Fluorophenyl)-3-hydroxymethyl-1-methylpiperidine
- CAS Number:105812-81-5
- Molecular formula:C13H18FNO
- Molecular Weight:223.29
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

105812-81-5
301 suppliers
$9.00/1g
Yield: 80%
Reaction Conditions:
Stage #1:(3S,4R)-3-ethoxycarbonyl-4-(4-fluorophenyl)-N-methylpiperidine-2,6-dione with lithium aluminium tetrahydride in tetrahydrofuran;toluene at 15 - 65;
Stage #2: with water;sodium hydroxide in tetrahydrofuran;toluene at 0 - 5; for 0.333333 h;
Steps:
4.3. (3S,4R)-trans-4-(4'-Fluorophenyl)-3-hydroxymethyl-N-methylpiperidine 7
Lithium aluminum hydride (1.8 g, 47.49 mmol) was added to a mixture of tetrahydrofuron (15.0 mL) and toluene (5.0 mL) at 0-5 °C and stirred for 15 min at the same temperature. A solution of (3S,4R)-3-ethoxycarbonyl-4-(4'-fluorophenyl)-N-methyl piperidine-2,6-dione 6 (5.0 g, 17.05 mmol) in toluene (15.0 mL) was added slowly for 45-60 min at below 15 °C and the mixture was heated to 60-65 °C for 2 h. The mixture was cooled to 0-5 °C and basified with 10% sodium hydroxide solution. Water (10.0 mL) was charged to the reaction mixture and then stirred for 20 min. The separated solid was filtered and washed with toluene (2 × 15 mL). The solvent was removed under reduced pressure and the crude compound was recrystalized from n-heptane to afford 7 (3.0 g, 80%) as an white solid. inlMMLBox (c 1, ethanol); IR (cm-1): 3153, 2943, 1602, 1509, 1222, 1379, 1069; 1H NMR: (400 MHz, CDCl3) δ, ppm: 7.14-7.17 (m, 2H), 6.96-7.0 (m, 2H), 3.38-3.42 (m, 1H), 3.13-3.25 (m, 2H), 2.92-2.95 (m, 1H), 2.29-2.34 (m, 4H), 1.97-2.03 (m, 5H); MS: m/z: 224.2 [M+H+].
References:
Somaiah, Sripathi;Sashikanth, Suthrapu;Raju, Veeramalla;Reddy, Karnati Venugopal [Tetrahedron Asymmetry,2011,vol. 22,# 1,p. 1 - 3] Location in patent:experimental part
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

105812-81-5
301 suppliers
$9.00/1g
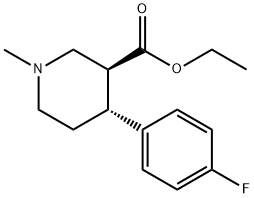
858938-15-5
0 suppliers
inquiry

105812-81-5
301 suppliers
$9.00/1g