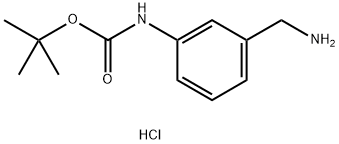
tert-Butyl (3-(aminomethyl)phenyl)carbamate hydrochloride synthesis
- Product Name:tert-Butyl (3-(aminomethyl)phenyl)carbamate hydrochloride
- CAS Number:1038549-44-8
- Molecular formula:C12H19ClN2O2
- Molecular Weight:258.75

1038549-44-8
3 suppliers
inquiry
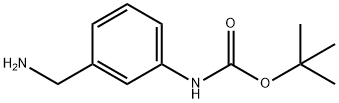
205318-52-1
94 suppliers
$25.00/250mg
Yield:-
Reaction Conditions:
with Si-dimethylamine in N,N-dimethyl-formamide; for 1 h;
Steps:
6
3-t-Butoxycarbonylamino-benzyl-ammonium chloride (103.5 mg, 0.4 mmol) was dissolved in 1 rnL DMF in a reaction vial, and Si-dimethylamine (350 mg, 0.525 mmol) was added. The resulting mixture was agitated for 1 h, after which time/?- cyanophenylisocyanate (52 mg, 0.36 mmol) was added and the reaction mixture was agitated 8 h. SCX (113 mg, 0.1 mmol) was added and the mixture was agitated an additional 30 minutes. The reaction mixture was filtered, the flrate was concentrated, and the residue was dissolved in 5 mL dichloromethane. TFA (1 mL) was added and the mixture was stirred at room temperature for 1.5 hours. The solvents were evaporated providing 96 mg of pure intermediate (l-(3-amino-benzyl)-3-(4-cyano-phenyl)-urea) which was used directly. Synthesis of the second reagent was carried out by treating l,2,3,4-tetrahydro-isoquinoline-7-carboxylic acid methyl ester (139 mg, 0.727 mmol) with formaldehyde (1 mL, 37% aqueous) in formic acid (1 mL) and heating the resulting
References:
WO2008/86047,2008,A1 Location in patent:Page/Page column 83-84