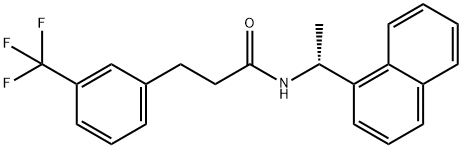
N-((R)-1-(naphthalen-1-yl)ethyl)-3-(3-(trifluoroMethyl)phenyl)propanaMide synthesis
- Product Name:N-((R)-1-(naphthalen-1-yl)ethyl)-3-(3-(trifluoroMethyl)phenyl)propanaMide
- CAS Number:1005450-55-4
- Molecular formula:C22H20F3NO
- Molecular Weight:371.4
![2-PropenaMide, N-[(1R)-1-(1-naphthalenyl)ethyl]-3-[3-(trifluoroMethyl)phenyl]-, (2E)-](/CAS/20150408/GIF/1095393-66-0.gif)
1095393-66-0
9 suppliers
inquiry

1005450-55-4
112 suppliers
$72.00/250mg
Yield: 91.2%
Reaction Conditions:
with hydrogen;palladium on activated carbon in methanol;toluene at 20; for 1.5 h;Product distribution / selectivity;
Steps:
2
hi yet another variation, the reduction of the double bond and amide carbonyl of (E)-N-((i?)-l-naphthalen-l-yl-ethyl)-3-(3-trifluoromethyl-phenyl)-acrylamide is carried out in a stepwise manner. Either the carbonyl group or the alkene double bond can be reduced first. For example, the reduction of the alkene double bond is achieved by hydrogenation in the presence of a metal catalyst.[0123] A suspension of (E)-N-((i?)-l-naphthalen-l-yl-ethyl)-3-(3-trifluoromethyl-phenyl)- acrylamide (1.0 equiv.; 0.67 mmol; 250 mg) and Pd/C 10 wt% (25 mg) was stirred in a mixture of methanol and toluene (7 mL/lmL). The reaction flask was evacuated and back filled using a hydrogen filled balloon (three times). The reaction mixture is stirred at room temperature and the progress of the reaction was monitored using high performance liquid chromatography. After 90 minutes, the reaction mixture was filtered over a bed of celite and the solvent from the filtrate was removed in vacuo. The resulting solid was dried overnight at 450C using a vacuum oven. Isolated yield: 91.2% (229 mg). The identity of the amide was confirmed by 1H NMR and mass spectrometry. (400MHz, CDCl3) δ ppm 1.60 (d, 3H) 2.39- 2.53 (m, 2H) 2.96-3.13 ( m, 2H) 5.55 (br. S., IH) 5.87-5.96 (m, IH) 7.29-7.38 (m, 2H) 7.39- 7.46 (m, 4H) 7.46-7.55 (m, 2H) 7.76-7.82 (m, IH) 7.83-7.89 (m, IH) 8.00-8.07 (m, IH) and HRMS (M+l): 372.1
References:
AMGEN INC. WO2009/2427, 2008, A2 Location in patent:Page/Page column 39
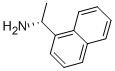
3886-70-2
510 suppliers
$5.00/1g
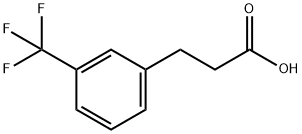
585-50-2
329 suppliers
$19.00/1g

1005450-55-4
112 suppliers
$72.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1005450-55-4
112 suppliers
$72.00/250mg

3886-70-2
510 suppliers
$5.00/1g
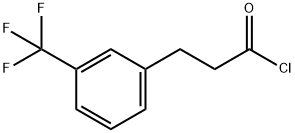
455-03-8
4 suppliers
inquiry

1005450-55-4
112 suppliers
$72.00/250mg

585-50-2
329 suppliers
$19.00/1g
82572-04-1
92 suppliers
$11.00/100mg

1005450-55-4
112 suppliers
$72.00/250mg