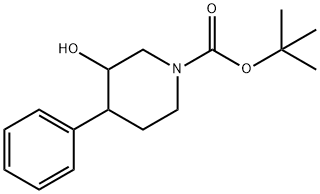
TERT-BUTYL 3-HYDROXY-4-PHENYLPIPERIDINE-1-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 3-HYDROXY-4-PHENYLPIPERIDINE-1-CARBOXYLATE
- CAS Number:1000931-04-3
- Molecular formula:C16H23NO3
- Molecular Weight:277.36
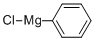
100-59-4
263 suppliers
$44.00/0.25mole
![7-Oxa-3-azabicyclo[4.1.0]heptane-3-carboxylic acid, 1,1-dimethylethyl ester](/CAS/GIF/161157-50-2.gif)
161157-50-2
125 suppliers
$9.00/250mg

1000931-04-3
39 suppliers
$45.00/10mg
Yield:1000931-04-3 94%
Reaction Conditions:
Stage #1: phenylmagnesium chloride;tert-butyl 7-oxa-3-azabicyclo[4.1.0]heptane-3-carboxylate;copper(l) iodide in tetrahydrofuran at -30 - 25; for 16 h;
Stage #2: with water;ammonium chloride in tetrahydrofuran;
Steps:
4
According to Tetrahedron Lett., 1979, 17, 1503, to a stirred solution of phenylmagnesium chloride (0.753 mL, 1.51 mmol) and copper (I) iodide (0.0191 g, 0.100 mmol) in THF (3 mL) at -300C was added tert-butyl 7-oxa-3-aza- bicyclo[4.1.0]heptane-3-carboxylate (0.200 g, 1.00 mmol) in THF (1 mL) . The mixture was allowed to warm to room temperature and was stirred for 16 h. It was then quenched with saturated aqueous ammonium chloride and the mixture was extracted with AcOEt. The combined organic layers were dried (sodium sulfate) , filtered, and concentrated in vacuo to give tert-butyl 3-hydroxy-4-phenylpiperidine-l-carboxylate (0.261 g, 94%) as an oil: 1H NMR (400 MHz, DMSO-d6) δ 1-49 (s, 9H), 1.76 (m, 3H), 2.50-2.78 (m, 3H), 3.70 (m, IH), 4.30 (bd, IH), 7.30 (m, 5H).
References:
WO2008/11130,2008,A2 Location in patent:Page/Page column 136-137
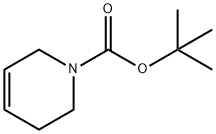
85838-94-4
228 suppliers
$7.00/1g

1000931-04-3
39 suppliers
$45.00/10mg
