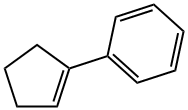
1-PHENYLCYCLOPENTENE synthesis
- Product Name:1-PHENYLCYCLOPENTENE
- CAS Number:825-54-7
- Molecular formula:C11H12
- Molecular Weight:144.21
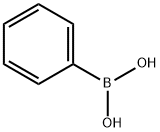
120-92-3
408 suppliers
$11.00/25g
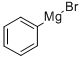
100-58-3
187 suppliers
$18.00/10ml

825-54-7
55 suppliers
$45.00/10mg
Yield: 100%
Reaction Conditions:
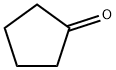
Stage #1:cyclopentanone;phenylmagnesium bromide in tetrahydrofuran;diethyl ether at 0;Reflux;
Stage #2: with hydrogenchloride in tetrahydrofuran;diethyl ether;water
Steps:
G.1
To a solution of 3.0 M solution of phenylmagnesium bromide in ether (49.7 mL, 149 mmol) was added TΗF (300 mL). To this solution cooled to 00C cyclopentanone(13.23 mL, 149 mmol) was added. The reaction mixture was stirred at room temperature for 30 min, then - at reflux for 2 h. Ice (20 g) was added, followed by 6N HCl, until the precipitate dissolved. The product was extracted with ether. The combined etherial layers were washed with saturated sodium bicarbonate solution, dried over anhydrous magnesium sulfate and filtered. The solvent was removed in vacuum and the residue was purified by column chromatography on silica gel to give cyclopentenylbenzene (21.49 g, 149 mmol, 100 % yield) as colorless oil. LC-MS (M+H)+ = 145.1. 1H NMR (500 MHz, CDCl3) δ ppm 7.48 (2H, d, J=7.3 Hz), 7.35 (2H, t, J=7.8 Hz), 7.22 - 7.27 (IH, m), 6.22 (IH, t, J=2.1 Hz), 2.70 - 2.80 (2H, m), 2.52 - 2.64 (2H, m), 2.01 - 2.12 (2H, m).
References:
BRISTOL-MYERS SQUIBB COMPANY;BOY, Kenneth M.;GUERNON, Jason M.;MACOR, John E.;OLSON, Richard E.;SHI, Jianliang;THOMPSON, III, Lorin A.;WU, Yong-Jin;XU, Li;ZHANG, Yunhui;ZUEV, Dmitry S. WO2011/14535, 2011, A1 Location in patent:Page/Page column 50-51
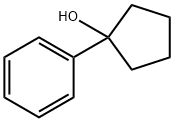
10487-96-4
31 suppliers
$33.39/250mg

825-54-7
55 suppliers
$45.00/10mg
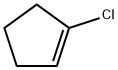
930-29-0
37 suppliers
$65.00/250mg
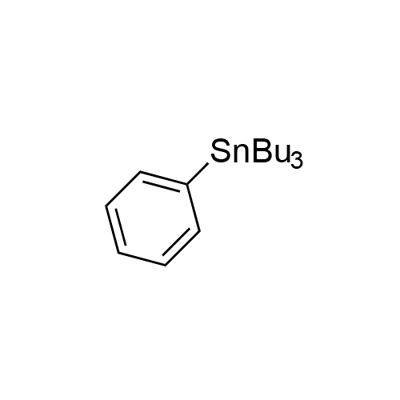
960-16-7
95 suppliers
$22.60/1g

825-54-7
55 suppliers
$45.00/10mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![9-Borabicyclo[3.3.1]nonane](/CAS/GIF/280-64-8.gif)
280-64-8
154 suppliers
$45.00/50ml

825-54-7
55 suppliers
$45.00/10mg