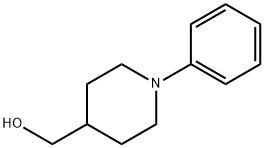
(1-Phenyl-4-piperidyl)Methanol synthesis
- Product Name:(1-Phenyl-4-piperidyl)Methanol
- CAS Number:697306-45-9
- Molecular formula:C12H17NO
- Molecular Weight:191.27
247022-37-3
21 suppliers
inquiry

697306-45-9
46 suppliers
$67.00/250mg
Yield:697306-45-9 99%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 2 h;
Steps:
294 Reference Example 294; (1-phenyl-piperidin-4-yl)-methanol
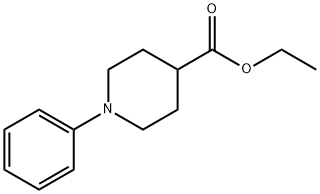
To a solution of ethyl 1-phenylpiperidine-4-carboxylate (2.6 g, 6.86 mmol) in THF. (32 mL) cooled to 0°C was added aluminum lithium hydride (325 mg, 8.58 mmol) and the mixture was stirred at 0°C for 2 hrs. To the reaction solution was added saturated aqueous ammonium chloride solution and insoluble materials were removed by decantation. The organic layer was dried over anhydrous magnesium sulfate and concentrated. The residue was purified by silica gel column chromatography (developing solvent: hexane/ethyl acetate = 4/1) to give the title compound as a colorless solid (1.3 g, 99%). 1H NMR (400 MHz, CDC13) 8 ppm : 1.25-1. 46 (m, 3 H), 1.62- 1.69 (m, 1 H), 1. 83-1. 88 (m, 2 H), 2.72 (td, J = 12.2, 2.4 Hz, 1 H), 3.55 (t, J = 5. 8 Hz, 2 H), 3. 69-3. 74 (m, 2 H), 6.81-6. 96 (m, 3 H), 7.23-7. 28 (m, 2 H).
References:
WO2004/46107,2004,A1 Location in patent:Page 227