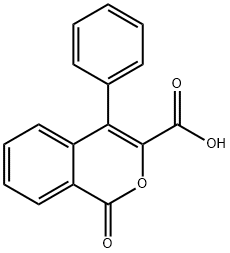
1-OXO-4-PHENYL-1H-ISOCHROMENE-3-CARBOXYLIC ACID synthesis
- Product Name:1-OXO-4-PHENYL-1H-ISOCHROMENE-3-CARBOXYLIC ACID
- CAS Number:37617-98-4
- Molecular formula:C16H10O4
- Molecular Weight:266.25
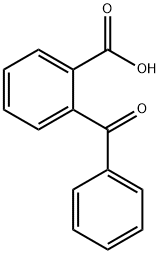
85-52-9
322 suppliers
$6.00/25g
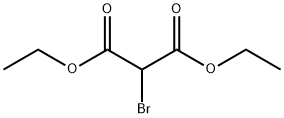
685-87-0
260 suppliers
$10.00/5g

37617-98-4
11 suppliers
$219.00/500mg
Yield:37617-98-4 84%
Reaction Conditions:
Stage #1: 2-Benzoylbenzoic acid;Diethyl 2-bromomalonatewith potassium carbonate in DMF (N,N-dimethyl-formamide) at 20;
Stage #2: with hydrogenchloride;acetic acid in water; for 6 h;Heating / reflux;
Steps:
5.1.2
5.1.2 PREPARATION OF 4-PHENYL-3-ISOCOUMARINCARBOXYLIC ACID (102): Following a literature procedure (Natsugary et al, J. Med. Chem. 1995, 38, 3106-3120), Compound 102 was synthesised. A suspension of 2-benzoyl-benzoic acid (33.9 g, 0.15 mol), anhydrous potassium carbonate (41.4 gm, 0.3 mol) and diethyl bromomalonate ((28.17 mL, 0.165 mol) in DMF (250 mL) was allowed to stir overnight at room temperature. The reaction mixture was then poured on cold water, and extracted with ethyl acetate. The organic layer was dried over sodium sulphate and concentrated. The residue obtained was treated with acetic acid (1.0 L), followed by concentrated HCl (800 mL), and then refluxed for 6 hours. The reaction mixture was cooled to room temperature and poured on ice cold water, and the precipitate that formed was filtered, washed thoroughly with water, and dried to provide 32.6 g of Compound 102, a white solid, in 84% yield.
References:
US2005/261288,2005,A1 Location in patent:Page/Page column 33
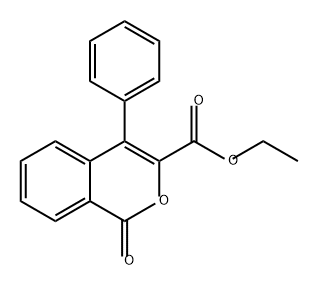
94224-71-2
0 suppliers
inquiry

37617-98-4
11 suppliers
$219.00/500mg