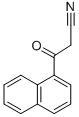
1-NAPHTHOYLACETONITRILE synthesis
- Product Name:1-NAPHTHOYLACETONITRILE
- CAS Number:39528-57-9
- Molecular formula:C13H9NO
- Molecular Weight:195.22
Yield:39528-57-9 72%
Reaction Conditions:
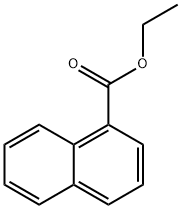
Stage #1: acetonitrilewith n-butyllithium in tetrahydrofuran;hexane at -78; for 0.5 h;
Stage #2: ethyl 1-naphthoate in tetrahydrofuran;hexane at -78 - 20; for 2 h;
Steps:
B.2b-B.1 Step 1:
1-Naphthoylacetonitrile
Step 1:
1-Naphthoylacetonitrile
To a solution of 2.50 M of n-butyllithium in hexane (49.9 mL, 125 mmol) in tetrahydrofuran (49.9 mL) cooled to -78° C. is added acetonitrile (6.52 mL, 125 mmol) drop-wise.
The resulting cloudy mixture is stirred for 30 min.
Ethyl α-napthoate (8.87 mL, 49.9 mmol) is added drop-wise and the reaction mixture is stirred at -78° C. for 2 hr.
The reaction is warmed to room temperature, quenched with acetic acid (50 ml) and partitioned between ethyl acetate and water.
The organic layer is dried over Na2SO4, filtered and concentrated in vacuo.
The crude material is purified by column chromatography (eluent: 5-15% CH2Cl2/methanol) to provide 1-naphthoylacetonitrile (6.99 g, yield 72%).
1H NMR (400 MHz, CDCl3) δ 8.82 (d, J=8.7 Hz, 1H), 8.11 (d, J=8.3 Hz, 1H), 7.90 (t, J=8.1 Hz, 2H), 7.71-7.64 (m, 1H), 7.57 (dt, J=15.5, 7.2 Hz, 2H), 4.21 (s, 2H).
References:
US2013/217682,2013,A1 Location in patent:Paragraph 0269; 0270
14271-86-4
43 suppliers
$60.00/100mg

590-17-0
327 suppliers
$5.00/5g

39528-57-9
35 suppliers
inquiry