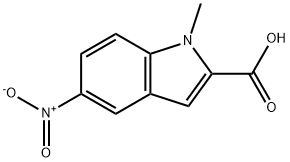
1-methyl-5-nitro-1H-indole-2-carboxylic acid synthesis
- Product Name:1-methyl-5-nitro-1H-indole-2-carboxylic acid
- CAS Number:71056-94-5
- Molecular formula:C10H8N2O4
- Molecular Weight:220.18

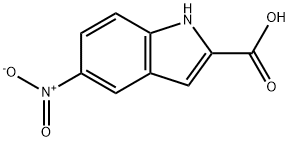
74-88-4
349 suppliers
$15.00/10g

71056-94-5
14 suppliers
inquiry
Yield:71056-94-5 45%
Reaction Conditions:
Stage #1: 5-nitro-indole-2-carboxylic acid ethyl esterwith sodium hydride in DMF (N,N-dimethyl-formamide);n-heptane at 20; for 0.166667 h;
Stage #2: methyl iodide in DMF (N,N-dimethyl-formamide);n-heptane; for 1 h;
Stage #3: with hydrogenchloride;water pH=1;
Steps:
9.s.i
(s) 1-Methyl-5-[(5-phenyl-furan-2-carbonyl)-amino]-1H- indole-2-carboxylic acid (118); (i) 1-Methyl-5-nitro-lH-indole-2-carboxylic acid; 5-Nitro-lH-indole-2-carboxylic acid ethyl ester (234 mg, 1 mmol) was dissolved in DMF (2 ml). NaH (32 mg, 1.4 mmol) was added as a suspension in heptane (0.5 ml) and the mixture was stirred at room temperature for 10 min. Iodomethane (170 mg, 1.2 mmol) was added drop-wise and the solution was left stirring for 1 h. During this reaction, hydrolysis occurred. The reaction mixture was quenched with H20 (2 ml) and washed with EtOAc (3 x 1 ml). The aqueous layer was acidified to pH 1 with conc. HC1 until a white precipitate formed and extracted with EtOAc (3 x 1 ml). These organic layer were combined, dried (MgS04), filtered and the solvent removed in vacuo to give the title compound. Yield: 99 mg, 45% ; LC/MS tr 1.24 min; MS (ES+) m/z (M+H) not present ; 1H NMR (400 MHz; DMSO) 5 4.10 (s, 3H), 7.52 (s, 1H), 7.81 (d, 1H), 8.16-8. 20 (m, 1H), 8.74 (d, 1H).
References:
WO2005/80367,2005,A1 Location in patent:Page/Page column 165-166