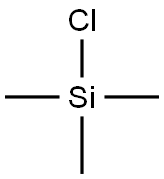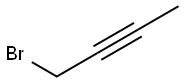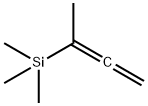
1-METHYL-1-(TRIMETHYLSILYL)ALLENE synthesis
- Product Name:1-METHYL-1-(TRIMETHYLSILYL)ALLENE
- CAS Number:74542-82-8
- Molecular formula:C7H14Si
- Molecular Weight:126.27

Yield:74542-82-8 83%
Reaction Conditions:
with methanesulfonyl chloride;copper(I) bromide;lithium bromide in tetrahydrofuran at -70 - 25;
References:
Danheiser, Rick L.;Carini, David J.;Kwasigroch, Carrie A. [Journal of Organic Chemistry,1986,vol. 51,# 20,p. 3870 - 3878]
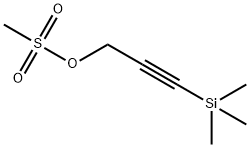
71321-17-0
0 suppliers
inquiry
676-58-4
296 suppliers
$15.00/25ml
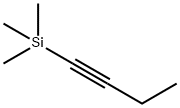
62108-37-6
56 suppliers
$11.00/100mg

74542-82-8
13 suppliers
inquiry
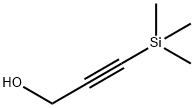
5272-36-6
197 suppliers
$6.00/1g

74542-82-8
13 suppliers
inquiry