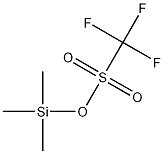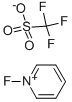
1-Fluoropyridinium triflate synthesis
- Product Name:1-Fluoropyridinium triflate
- CAS Number:107263-95-6
- Molecular formula:C6H5F4NO3S
- Molecular Weight:247.17

110-86-1
853 suppliers
$9.00/5g
1493-13-6
625 suppliers
$12.00/5g

107263-95-6
135 suppliers
$11.00/1g
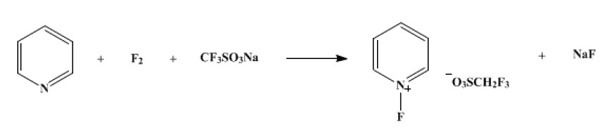
Yield:107263-95-6 96%
Reaction Conditions:
with fluorine in acetonitrile at -20; for 3 h;
References:
Umemoto, Teruo;Harasawa, Kikuko;Tomizawa, Ginjiro;Kawada, Kosuke;Tomita, Kyoichi [Bulletin of the Chemical Society of Japan,1991,vol. 64,# 4,p. 1081 - 1092]

110-86-1
853 suppliers
$9.00/5g
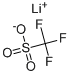
33454-82-9
357 suppliers
$6.00/5g

107263-95-6
135 suppliers
$11.00/1g

110-86-1
853 suppliers
$9.00/5g
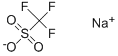
2926-30-9
273 suppliers
$11.00/5g

107263-95-6
135 suppliers
$11.00/1g

110-86-1
853 suppliers
$9.00/5g
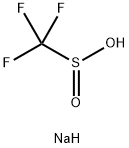
2926-29-6
294 suppliers
$6.00/5g

107263-95-6
135 suppliers
$11.00/1g