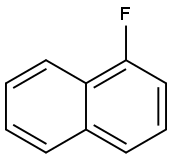
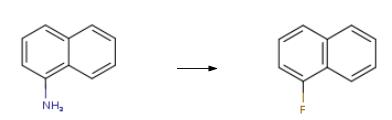
1-Fluoronaphthalene synthesis
- Product Name:1-Fluoronaphthalene
- CAS Number:321-38-0
- Molecular formula:C10H7F
- Molecular Weight:146.16
134-32-7
382 suppliers
$9.00/1g

321-38-0
606 suppliers
$6.00/10g
Yield:321-38-0 99.8%
Reaction Conditions:
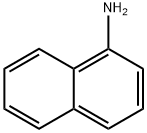
Stage #1: 1-amino-naphthalenewith hydrogenchloride;sodium nitrite in water at 5 - 75;
Stage #2: with tetrafluoroboric acid in water; for 0.25 h;
Stage #3: at 85 - 90;Temperature;
Steps:
1.1-1.4; 2.1-2.4; 3.1-3.4; 4.1-4.4; 5.1-5.4 Example 2
Preparation method of naphthalene-based fluorine-containing intermediate 1-fluoronaphthalene of the invention:1) Diazotization reaction: 1500 g of hydrochloric acid (mass concentration: 25%) and 300 g of naphthylamine were added to a 3000 mL three-necked flask, stirred and heated to 75 ° C to dissolve, and the temperature was lowered to below 5 ° C, and 148 g was slowly added at this temperature. Sodium nitrite, stirred at low temperature for 0.3 hours after the addition, to obtain a diazonium salt solution;2) Substitution reaction: 360 g of fluoroboric acid solution (concentration: 45%) was added to the resulting solution obtained in the step 1), stirred for 0.25 h, filtered, and the filter cake was dried at a temperature of 50 ° C for 0.2 h to obtain Dry naphthylamine diazonium salt fluoroborate double salt;3) Hot air decomposition: the dried diazonium salt fluoroborate double salt is slowly added to the reactor through which hot air (hot air temperature is 85-90 ° C), and the dried powdered naphthylamine diazonium salt fluoroborate double salt is The hot air blows up the dispersion and absorbs the heat for thermal decomposition to obtain a 1-fluoronaphthalene solution containing a small amount of solid impurities;4) Purification treatment: the 1-fluoronaphthalene solution obtained in the step 3) is first washed with pure water for 3 to 6 times, then neutralized with a soda ash to a pH of 6.8 to 7.2, and finally the oil layer is separated by filtration, and the filtrate is taken. The distillation treatment gave 210 g of a naphthalene-based fluorine-containing intermediate 1-fluoronaphthalene in an amount of 99.8%.
References:
CN109180416,2019,A Location in patent:Paragraph 0012; 0025-0054
166328-07-0
59 suppliers
$13.00/250mg

321-38-0
606 suppliers
$6.00/10g
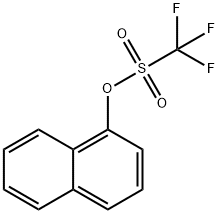
99747-74-7
52 suppliers
$16.00/250mg

321-38-0
606 suppliers
$6.00/10g
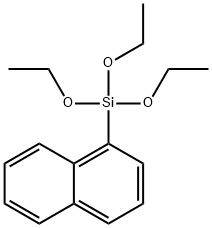
17938-06-6
59 suppliers
$35.00/5g

321-38-0
606 suppliers
$6.00/10g
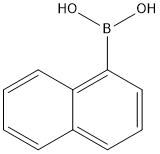
13922-41-3
491 suppliers
$5.00/1g

321-38-0
606 suppliers
$6.00/10g