
1-Eicosanol synthesis
- Product Name:1-Eicosanol
- CAS Number:629-96-9
- Molecular formula:C20H42O
- Molecular Weight:298.55
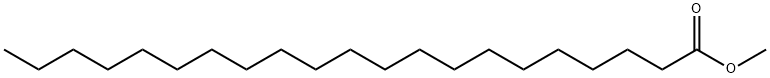
6064-90-0
109 suppliers
$39.00/50mg

629-96-9
209 suppliers
$14.00/5g
Yield:-
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20;
Steps:
1.2. General procedure for the synthesis of alkylglycerols (5a-5o)
General procedure: The synthesis of the AKGs was performed following the literature with some modifications (Baumann and Mangold 1964; Hanus et al. 2001; Appendino et al. 2003; Parkkari et al. 2006). LiAlH4 (550 mg, 14.5 mmol) was added slowly to a solution of the corresponding methyl ester (3.4 mmol) in anhydrous tetrahydrofuran (15 mL) at 0°C and stirred for 1 h and was then left at 20°C for 20 h. The reaction mixture was washed with NaOH (10%) followed by HCl (10%) and extracted with diethyl ether (3 × 20 mL); the extract was neutralized with saturated NaHCO3, dried under reduced pressure and purified by CC. The alcohol obtained was subsequently mesylated in absolute pyridine (4.5 mL, 55.6 mmol) at 0°C by the addition of MsCl (880 mg, 7.65 mmol) and the solution was maintained for 24 h at 20°C. After quenching the reaction with 5 mL of degasified water, the solution was extracted with diethyl ether (4 × 20 mL). The organic phase was washed with H2SO4 (2 N), neutralized, concentrated in vacuo, and the crude mesylate was purified by CC. KOH (127 mg, 2.26 mmol) was added to the chiral precursor (R)-solketal (283 mg, 2.14 mmol) in anhydrous toluene (2 mL). The reaction stirred at 50°C for 1 h before the addition of metallic Na (3 mg, 0.15 mmol) followed by the mesylate dissolved in toluene (15 mL), and the resulting mixture was kept at 50°C for 72 h. The reaction was quenched with HCl (10%) and extracted with ethyl ether (4 × 20 mL). The organic phase was neutralized, concentrated and purified by CC to give 1-O-alkyl-2,3-O-isopropylidene-sn-glycerol. This intermediate was deprotected in 5 mL of HCl:MeOH (1:10 v/v) and refluxed overnight. After the addition of H2O (10 mL) and extraction with diethyl ether (4 × 20 mL), the organic phase was neutralized, evaporatedto dryness in vacuo, and the residue was purified by CC to afford pure AKGs. Each step in the synthesis was monitored by TLC, and all CC steps were eluted with hexane-toluene-ethyl acetate (10:0:0-0:10:0-0:0:10) mixtures. The structures of the synthesised compounds were confirmed by 1H, 13C APT NMR with COSY, HMQC, HMBC and ESI-MS or HRESI-MS spectra along with the specific optical rotation.
References:
Fernández Montoya, Deicy J.;Contreras Jordan, Luis A.;Moreno-Murillo, Bárbara;Silva-Gómez, Edelberto;Mayorga-Wandurraga, Humberto [Natural Product Research,2019] Location in patent:supporting information

638-66-4
98 suppliers
$80.00/5g

629-96-9
209 suppliers
$14.00/5g
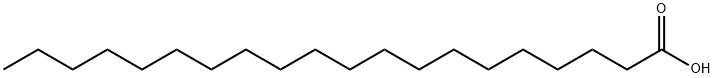
506-30-9
344 suppliers
$13.00/1g

629-96-9
209 suppliers
$14.00/5g
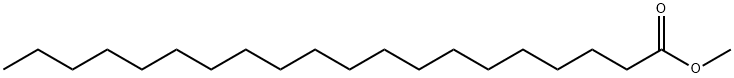
1120-28-1
204 suppliers
$6.00/100mg

629-96-9
209 suppliers
$14.00/5g
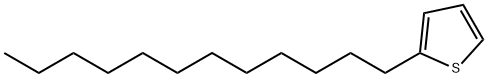
4861-61-4
28 suppliers
$49.00/1g

629-96-9
209 suppliers
$14.00/5g