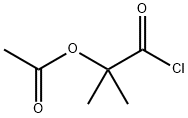
1-Chlorocarbonyl-1-methylethyl acetate synthesis
- Product Name:1-Chlorocarbonyl-1-methylethyl acetate
- CAS Number:40635-66-3
- Molecular formula:C6H9ClO3
- Molecular Weight:164.59
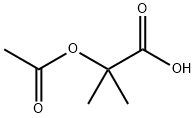
15805-98-8
26 suppliers
inquiry

40635-66-3
180 suppliers
$12.00/5g
Yield:-
Reaction Conditions:
with oxalyl dichloride in dichloromethane at 20; for 0.5 h;
Steps:
59.2 Step 2: Preparation of l-(7-bromo-2,3-dihydro-lH-pyrido[2,3-b][l,4]oxazin-l-yl)-2-methyl- l-oxopropan-2-yl acetate
To a solution of 2-acetoxy-2-methylpropanoic acid (1.09 g, 7.44 mmol) in DCM (6 mL), DMF (68 mg, 0.93 mmol) was added oxalyl chloride (0.814 mL, 9.30 mmol). The reaction mixture was stirred at 10 °C for 0.5 hour. A light yellow solution was formed. The mixture was concentrated to give crude l-chloro-2-methyl-l-oxopropan-2-yl acetate as a yellow oil. To a solution of 7-bromo-2,3-dihydro-lH-pyrido[2,3-b][l,4]oxazine (100 mg, 0.465 mmol) in DCM (6 mL) was added EhN (1.29 mL, 9.30 mmol). The reaction mixture was cooled to 0 °C and then dropwise added to the crude product l-chloro-2-methyl-l- oxopropan-2-yl acetate in DCM (4 mL). The reaction mixture was then warmed to 10 °C, stirred at 10 °C for 12 h under N2 atmosphere. The colorless solution turned to an orange solution gradually. LCMS (Rt = 0.614 min; MS Calcd: 342.0; MS Found: 342.9 [M+H]+). The reaction mixture was concentrated. The residue was purified by Combi Flash (1% EbN in DCM) to afford l-(7-bromo-2,3-dihydro-lH-pyrido[2,3-b][l,4]oxazin-l-yl)-2-methyl-l- oxopropan-2-yl acetate (320 mg, yield: 50% over three steps) as yellow oil.
References:
PETRA PHARMA CORPORATION;KESICKI, Edward A.;LINDSTR?M, Johan;PERSSON, Lars Boukharta;VIKLUND, Jenny;FORSBLOM, Rickard;GINMAN, Tobias;HICKEY, Eugene R.;DAHLGREN, Markus K.;GERASYUTO, Aleksey I. WO2019/126730, 2019, A1 Location in patent:Paragraph 0361
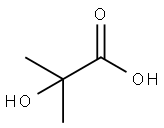
594-61-6
344 suppliers
$5.00/5g
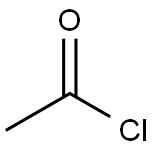
75-36-5
589 suppliers
$17.92/100G

40635-66-3
180 suppliers
$12.00/5g

594-61-6
344 suppliers
$5.00/5g
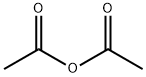
108-24-7
0 suppliers
$14.00/250ML

40635-66-3
180 suppliers
$12.00/5g