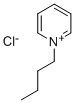
1-Butylpyridinium chloride synthesis
- Product Name:1-Butylpyridinium chloride
- CAS Number:1124-64-7
- Molecular formula:C9H14ClN
- Molecular Weight:171.67

110-86-1
848 suppliers
$9.00/5g

109-69-3
425 suppliers
$20.00/100ml

1124-64-7
205 suppliers
$10.00/1g
Yield:1124-64-7 88%
Reaction Conditions:
at 145;
Steps:
1
Example 1; The Preparation of N-Butyl-Pyridinium Chloroaluminate Ionic Liquid; N-butyl-pyridinium chloroaluminate is a room temperature ionic liquid prepared by mixing neat N-butyl-pyridinium chloride (a solid) with neat solid aluminum trichloride in an inert atmosphere. The syntheses of butylpyridinium chloride and the corresponding N-butyl-pyridinium chloroaluminate are described below. In a 2-L Teflon-lined autoclave, 400 gm (5.05 mol.) anhydrous pyridine (99.9% pure purchased from Aldrich) were mixed with 650 gm (7 mol.) 1-chlorobutane (99.5% pure purchased from Aldrich). The neat mixture was sealed and let to stir at 145° C. under autogenic pressure over night. Then, the autoclave was cooled down to room temperature, vented and the resultant mixture was transferred to a three liter round bottom flask. Chloroform was used to rinse the liner and dissolve the stubborn crusty product that adhered to the sides of the liner. Once all transferred, the mixture was concentrated at reduced pressure on a rotary evaporator (in a hot water bath) to remove excess chloride, un-reacted pyridine and the chloroform rinse. The obtained tan solid product was further purified by dissolving in hot acetone and precipitating the pure product through cooling and addition of diethyl ether. Filtering and drying under vacuum and heat on a rotary evaporator gave 750 gm (88% yields) of the desired product as an off-white shinny solid.
References:
US2006/135839,2006,A1 Location in patent:Page/Page column 3
4185-69-7
65 suppliers
$65.00/100mg

109-73-9
448 suppliers
$9.00/1g

1124-64-7
205 suppliers
$10.00/1g